|
captan
Fungicide
FRAC M4; multi-site: phthalimide
NOMENCLATURE
Common name captan (BSI, E-ISO, JMAF); captane ((m) F-ISO); captab (Republic of South Africa)
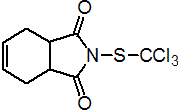
IUPAC name N-(trichloromethylthio)cyclohex-4-ene-1,2-dicarboximide
Chemical Abstracts name 3a,4,7,7a-tetrahydro-2-[(trichloromethyl)thio]-1H-isoindole-1,3(2H)-dione
CAS RN [133-06-2] EEC no. 205-087-0 Development codes SR 406 Official codes ENT 26 538
PHYSICAL CHEMISTRY
Composition Tech. is 90-95% pure. Mol. wt. 300.6 M.f. C9H8Cl3NO2S Form Colourless crystals; (tech. is a colourless to beige amorphous solid, with a pungent odour). M.p. 178 ºC; (tech., 175-178 ºC) V.p. <1.3 mPa (25 ºC) KOW logP = 2.8 (25 ºC) S.g./density 1.74 (26 °C) Solubility In water 3.3 mg/l (25 ºC). In xylene 20, chloroform 70, acetone 21, cyclohexanone 23, dioxane 47, benzene 21, toluene 6.9, isopropanol 1.7, ethanol 2.9, diethyl ether 2.5 (all in g/kg, 26 ºC). Insoluble in petroleum oils. Stability Hydrolysed slowly at neutral pH, rapidly in alkali. Thermal DT50 >4 y (80 ºC), 14.2 d (120 ºC).
COMMERCIALISATION
History Fungicide reported by A. R. Kittleston (Science, 1952, 115, 84). Introduced by Standard Oil Development Co., later by Chevron Chemical Co. Patents US 2553770; US 2553771; US 2553776 Manufacturers Arvesta; Crystal; Drexel; Makhteshim-Agan; Rallis; Sharda
APPLICATIONS
Biochemistry Non-specific thiol reactant, inhibiting respiration. Mode of action Fungicide with protective and curative action. Uses Control of a wide range of fungal diseases, e.g. scab, black rot, botryosphaeria rot, bitter rot, bull's eye rot, botrytis rot of pome fruit; shot-hole of stone fruit; peach leaf curl; brown rots of cherries, apricots, peaches, plums, and citrus fruit; downy mildew and black rot of vines; early and late blights of potatoes and tomatoes; Alternaria blight and leaf spot of carrots; anthracnose and downy mildew of cucurbits; leaf spot diseases of ornamentals; anthracnose and leaf spot diseases of tomatoes; brown patch on turf; Botrytis spp. on many crops; etc. Used on a large number of other crops. Applied at 2-4.5 lb/a. Used as a seed treatment or root dip for control of Pythium, Phoma, Rhizoctonia spp., etc. on maize, ornamentals, vegetables, oilseed rape, and other crops. Phytotoxicity Non-phytotoxic if used as directed, but some varieties of apple (e.g. Red Delicious, Winesap) and pear (e.g. D'Anjou, Bosc) may be injured, as also may lettuce seeds and, at higher dosages, celery and tomato seeds. Formulation types DP; DS; WG; WP; WS; SC. Compatibility Incompatible with alkaline materials, oil sprays, TEPP, and emulsifiable concentrate formulations of parathion. Selected products: 'Capone 480' (Ingeniería Industrial); 'Captaf' (Rallis); 'Captec' (Micro Flo); 'Criptan' (Vapco); 'Dhanutan' (Dhanuka); 'Lucaptan' (Lucava); 'Merpan' (Makhteshim-Agan); mixtures: 'Bayleton CA' (+ triadimefon) (Bayer CropScience); 'Rondo' (+ pyrifenox) (Syngenta)
OTHER PRODUCTS
'Captab' (Rallis); 'Captane' (Rallis); 'Captano' (Rallis); 'Captazel' (Arvesta); 'Kaptan' (Azot); 'Maestro' (Arvesta); 'Malvin' (Arvesta); 'Mercap' (Papaeconomou); 'Orthocide' (Arvesta); 'Sepicap' (Griffin); 'Sigma' (Arvesta); 'Sorene' (BASF); 'Tomo-okishiran' (Nihon Nohyaku) mixtures: 'Agrox D-L Plus' (+ diazinon+ gamma-HCH) (Agriliance); 'Agrox Premiere' (+ diazinon+ gamma-HCH+ metalaxyl) (Agriliance); 'Alpha Raxil CA' (+ tebuconazole+ anthraquinone) (seed treatment, France) (Bayer CropScience); 'Daipower' (+ iminoctadine tris(albesilate)) (Dainippon); 'Kernel Guard' (+ diazinon+ gamma-HCH) (Trace); 'Topas C' (+ penconazole) (Syngenta); 'Vitavax M DC' (+ carboxin) (Helena) Discontinued products: 'Captec' * (Griffin); 'Phytocape' * (Bayer) mixtures: 'Ciluan' * (+ pyridinitril) (E. Merck); 'Murcicide' * (+ phenylmercury nitrate) (Steetley)
ANALYSIS
Product analysis by glc (CIPAC Handbook, 1985, 1C, 2015), by hplc (AOAC Methods, 17th Ed., 980.06; CIPAC Handbook, 1985, 1C, 2013), by i.r. spectrometry or by total chlorine content after alkaline hydrolysis (ibid., 1970, 1, 171). Residues determined by glc (A. Ambrus et al., J. Assoc. Off. Anal. Chem., 1981, 64, 733; M. A. Luke et al., ibid., p. 1187; J. N. Ospenson, Anal. Methods Pestic., Plant Growth Regul. Food Addit., 1964, 3, 7; Anal. Methods Pestic. Plant Growth Regul., 1972, 6, 546) or by spectrophotometry of a derivative (AOAC Methods, 17th Ed., 957.14*).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 74, 76, 89 (see part 2 of the Bibliography). IARC ref. 30 class 3 Oral Acute oral LD50 for rats 9000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >4500 mg/kg. Corrosive to eyes; mild skin irritant (rabbits). Inhalation LC50 for rats 0.668 mg/l (tech.). Dust may cause respiratory irritation. NOEL (2 y) for rats 2000, dogs 4000 mg/kg diet. No carcinogenic, teratogenic, or mutagenic effects observed. ADI (JMPR) 0.1 mg/kg b.w. [1995, 2000]. Toxicity class WHO (a.i.) U; EPA (formulation) IV EC classification R40| T; R23| Xi; R41| R43| N; R50
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks and pheasants >5000, bobwhite quail 2000-4000 mg/kg. Not toxic to starlings or red-winged blackbirds at 100 mg/kg. Fish LC50 (96 h) for bluegill sunfish 0.072, harlequin fish 0.3, brook trout 0.034 mg/l. Daphnia LC50 (48 h) 7-10 mg/l. Other aquatic spp. Moderately toxic to aquatic invertebrates. Bees LD50 (oral) 91; (contact) 788 mg/bee.
ENVIRONMENTAL FATE
Animals In mammals, the three chlorine atoms are cleaved under the influence of cellular sulfhydryl compounds. Trithiocarbamate, thiophosgene, and tetrahydrophthalimide are formed (R. G. Owens, Ann. New York Acad. Sci., 1969, 160, 114-132). Soil/Environment Soil Kd 3-8 (pH 4.5-7.2, o.m. 2.2-6.7%); DT50 c. 1 d (25 ºC, pH 7.2, o.m. 1.2%).
|