|
zoxamide
Fungicide
FRAC 22, B3; benzamide (F)
NOMENCLATURE
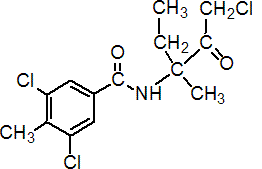
Common name zoxamide (BSI, pa ISO)
IUPAC name (RS)-3,5-dichloro-N-(3-chloro-1-ethyl-1-methyl-2-oxopropyl)-p-toluamide
Chemical Abstracts name 3,5-dichloro-N-(3-chloro-1-ethyl-1-methyl-2-oxopropyl)-4-methylbenzamide
CAS RN [156052-68-5] Development codes RH-7281; RH-117281 (both Rohm & Haas)
PHYSICAL CHEMISTRY
Mol. wt. 336.6 M.f. C14H16Cl3NO2 Form Fine, white powder, with a liquorice-like odour. M.p. 159.5-161 °C V.p. <1 ´ 10-2 mPa (up to 45 °C) KOW logP = 3.76 (20 °C) S.g./density 1.38 (20 °C) Solubility In water 0.681 mg/l (20 °C). In acetone 55.7 g/l (25 °C). Stability Aqueous hydrolysis DT50 c. 15 d (pH 4 and 7), c. 8 d (pH 9). Aqueous photolysis DT50 7.8 d.
COMMERCIALISATION
History Reported by A. R. Egan et al. (Proc. Br. Crop Prot. Conf. - Pests Dis., 1998, 2, 335). Developed by Rohm & Haas Co. (now Dow AgroSciences), first registered in USA and launched in Europe in 2001. Patents US 5304572 Manufacturers Dow AgroSciences
APPLICATIONS
Biochemistry Prevents nuclear division by binding to the b-subunit of tubulin and disruption of the microtubule cytoskeleton. Mode of action Preventative fungicide with rainfast and residual properties. Uses Applied at 120-200 g/ha, provides control of Oomycete fungi, including Phytophthora infestans, on potatoes (foliar, stem, tuber blight) and tomatoes, Plasmopara viticola on vines and Pseudoperonospora cubensis on cucurbits. Zoxamide is not cross-resistant to current Oomycete fungicides, such as phenylamides, QoI fungicides or cymoxanil. Sold in preformulated mixtures with either mancozeb or cymoxanil. Formulation types WG; WP. Selected products: 'Zoxium' (Dow AgroSciences); mixtures: 'Electis' (+ mancozeb) (Dow AgroSciences); 'Gavel' (+ mancozeb) (Dow AgroSciences); 'Roxam' (+ mancozeb) (Interfarm)
OTHER PRODUCTS
Mixtures: 'Aderio' (+ mancozeb) (Dow AgroSciences); 'Harpon' (+ cymoxanil) (Dow AgroSciences); 'Stimo' (+ mancozeb) (Dow AgroSciences); 'Unikat' (+ mancozeb) (Dow AgroSciences)
ANALYSIS
Product analysis by reverse phase hplc with uv detection. Residues of zoxamide in crops and various environmental matrices are analysed by gc with ECD detection. Details available from Dow AgroSciences.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 >2000 mg/kg. Non-irritating to skin; moderate eye irritant (rabbits) in USA, no classification in EU. Is a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >5.3 mg/l. NOEL Dietary NOEL (1 y) for dogs 50 mg/kg b.w. daily. ADI 2.6 mg/kg b.w. (proposed) Other Negative in 4 mutagenicity tests, non-teratogenic, no adverse reproductive effects; no oncogenic potential.
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Dietary LC50 for mallard ducks and bobwhite quail >5250 mg/kg diet. Reproduction NOEL for mallard ducks and bobwhite quail 1000 mg/kg diet. Fish LC50 (96 h) for rainbow trout 160 mg/l; LC50 (96 h) for bluegill sunfish >790, sheepshead minnow >855, zebra fish >730 mg/l (all greater than solubility limit). Life cycle NOEC for fathead minnow 60 mg/l. Daphnia EC50 (48 h) >780 mg/l (solubility limit). Reproduction NOEC (21 d) 39 mg/l. Algae EC50 (120 h, cell density) for Selenastrum capricornutum 19, Scenedesmus subspicatus 11 mg/l; EC50 (120 h, cell density) for Anabaena flos-aquae >860, Navicula pelliculosa >930, Skeletonema costatum >910 mg/l (all greater than solubility limit). Other aquatic spp. EC50 (48 h) for Eastern oyster 703 mg/l; LC50 (96 h) for mysid shrimp 76 mg/l. EC50 (14 d) for Lemna gibba 17 mg/l. Bees LD50 (contact) for honeybees >100 mg/bee. Worms LC50 (14 d) >1070 mg/kg soil; sublethal growth and reproduction NOEC 7 mg/kg natural soil. Other beneficial spp. IOBC classification: Harmless at 0.15 kg/ha (1´ typical rate) to Typhlodromus pyri, Aphidius rhopalosiphi, Amblyseius andersoni, Pardosa sp., Poecilus cupreus, Chrysoperla carnea, Orius insidiosus; slightly harmful at 0.3 kg/ha (2´ typical rate) to Aphidius and Chrysoperla.
ENVIRONMENTAL FATE
Zoxamide is a relatively transient molecule in the environment with low potential for persistence or movement. Animals In metabolism studies faecal excretion was the primary route of elimination. A total of 36 metabolites, including the parent compound, were detected in the urine and faeces; the parent compound was the principal component excreted. Bioconcentration factor in bluegill sunfish 95-136; 50% clearance time 0.4 days. Plants Zoxamide penetrates surface layers of the plant but has limited translocation. Soil/Environment Soil DT50 2-10 d; CO2 is the major metabolite. Koc 1166-1224; low mobility, no leaching potential.
|