|
uniconazole
Plant growth regulator
NOMENCLATURE
uniconazole
Common name uniconazole (BSI, ANSI, draft E-ISO, (m) draft F-ISO)
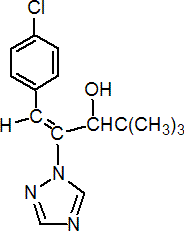
IUPAC name (E)-(RS)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pent-1-en-3-ol
Chemical Abstracts name (E)-(?-b-[(4-chlorophenyl)methylene]-a-(1,1-dimethylethyl)-1H-1,2,4-triazole-1-ethanol
CAS RN [83657-22-1] Development codes S-07; S-327 (both Sumitomo); XE-1019 (Chevron)
uniconazole-P
Common name uniconazole-P (BSI, pa E-ISO)
IUPAC name (E)-(S)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pent-1-en-3-ol
CAS RN [83657-17-4] (E)-(S)-(+)- isomer; [83657-16-3] (E)-(R)-(-)- isomer; [76714-83-5] (E)- isomers Development codes S-3307 D (Sumitomo)
PHYSICAL CHEMISTRY
uniconazole
Mol. wt. 291.8 M.f. C15H18ClN3O Form White, crystalline solid. M.p. 147-164 ºC V.p. 8.9 mPa (20 ºC) KOW logP = 3.67 (25 ºC) S.g./density 1.28 (21.5 ºC) Solubility In water 8.41 mg/l (25 ºC). In methanol 88, hexane 0.3, xylene 7 (all in g/kg, 25 ºC). Soluble in acetone, ethyl acetate, chloroform, and dimethylformamide. Stability Good stability under normal storage conditions.
uniconazole-P
Mol. wt. 291.8 M.f. C15H18ClN3O Form White, crystalline solid, with a faint characteristic odour. M.p. 152.1-155.0 ºC V.p. 5.3 mPa (20 ºC) S.g./density 1.28 (25 ºC). Solubility In water 8.41 mg/l (25 ºC). In methanol 72, hexane 0.2 (both in g/l, 20 ºC). Stability Stable under normal storage conditions. F.p. 195 ºC
COMMERCIALISATION
History Plant growth regulator reported by K. Izumi et al. (Plant Cell Physiol., 1984, 25, 611); uniconazole-P is more potent than the (R)-(-)- isomer. Introduced by Sumitomo Chemical Co., Ltd and by Valent.
uniconazole
Patents US 4203995; GB 2004276 Manufacturers Sumitomo
uniconazole-P
Patents US 4435203
APPLICATIONS
Biochemistry Inhibits gibberellin biosynthesis. Mode of action Plant growth regulator, absorbed by the stems and roots, with translocation in the xylem to growing points. Uses Used to reduce lodging in rice; to reduce vegetative growth and increase flowering of ornamentals; and to reduce vegetative growth and the need for pruning in trees. Formulation types GR; SL; WP.
uniconazole
Selected products: 'Lomica' (Japan) (Sumitomo); 'Sumiseven' (Japan) (Sumitomo)
uniconazole-P
Selected products: 'Sumagic' (Sumitomo, Valent)
OTHER PRODUCTS
uniconazole-P
'Prunit' (Sumitomo); 'Sunny' (Aquamarine)
ANALYSIS
Product analysis by glc or hplc; details available from Sumitomo. Residues determined by glc or hplc.
MAMMALIAN TOXICOLOGY
uniconazole-P
Oral Acute oral LD50 for male rats 2020, female rats 1790 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Not irritating to skin; minimal eye irritation (rabbits). Inhalation LC50 (4 h) for rats >2750 mg/m3. Other Non-mutagenic in Ames assay. Toxicity class WHO (a.i.) III (classification assigned to uniconazole on the basis of data for uniconazole-P); EPA (formulation) III
ECOTOXICOLOGY
uniconazole-P
Fish LC50 (96 h) for rainbow trout 14.8, carp 7.64 mg/l. Bees Acute contact LD50 for bees >20 mg/bee.
|