|
triticonazole
Fungicide
FRAC 3, G1; DMI: triazole
NOMENCLATURE
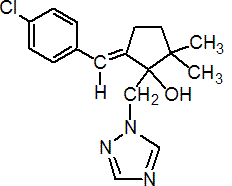
Common name triticonazole (BSI, pa E-ISO)
IUPAC name (?-(E)-5-(4-chlorobenzylidene)-2,2-dimethyl-1-(1H-1,2,4-triazol-1-ylmethyl)cyclopentanol
Chemical Abstracts name 5-[(4-chlorophenyl)methylene]-2,2-dimethyl-1-(1H-1,2,4-triazol-1-ylmethyl)cyclopentanol
CAS RN [131983-72-7] Development codes RPA 400727 (Rhône-Poulenc)
PHYSICAL CHEMISTRY
Composition Material is a racemic mixture; 95% pure. Mol. wt. 317.8 M.f. C17H20ClN3O Form White powder, odourless at 22 ºC. M.p. 139-140.5 ºC V.p. <1 ´ 10-5 mPa (50 ºC) KOW logP = 3.29 (20 ºC) Henry <3.9 ´ 10-5 Pa m3 mol-1 (calc.) S.g./density 1.326-1.369 (20 ºC) Solubility In water 9.3 mg/l (20 ºC), independent of pH. Stability Slight decomposition at 180 ºC.
COMMERCIALISATION
History Fungicide developed by Rhône-Poulenc Agrochimie (now Bayer CropScience) and first registered in France in 1993. Some rights, primarily in Europe, acquired by BASF AG in 2003. Patents FR 2641277 (1988) Manufacturers BASF
APPLICATIONS
Biochemistry Inhibition of sterol demethylation. Uses Seed disinfectant against seed-borne diseases, and a preventive treatment against a number of foliar pathogens such as rusts, powdery mildew, leaf spot, eyespot, leaf and net blotch of cereals, and head smut of maize; applied as a seed treatment at 50 g/t for cereals and 200 g/t for maize. Formulation types FS. Selected products: 'Real' (Bayer CropScience, BASF)
OTHER PRODUCTS
'Alios' (Bayer CropScience, BASF); 'Charter' (Bayer CropScience, BASF) mixtures: 'Jumper' (+ fipronil+ guazatine) (BASF); 'Zoom' (+ fipronil+ guazatine) (BASF); 'Kinto' (+ anthraquinone+ prochloraz) (Bayer CropScience, BASF); 'Legat' (+ guazatine acetates) (seed treatment) (Makhteshim-Agan); 'Premis 25' (+ cypermethrin) (Bayer CropScience, BASF); 'Premis B' (+ guazatine) (Bayer CropScience, BASF); 'Premis Geta' (+ guazatine acetates) (Bayer CropScience, BASF); 'Premis Or' (+ anthraquinone+ iprodione) (Bayer CropScience, BASF); 'Premis' (+ guazatine acetates) (Bayer CropScience, BASF); 'Robust' (+ imazalil) (Bayer CropScience, BASF)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >2000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Not a skin or eye irritant. Inhalation LC50 >1.4 mg/l air. NOEL Chronic NOEL for rats 750 ppm (29.4 and 38.3 mg/kg b.w. daily for males and females, respy.); for dogs 2.5 mg/kg b.w. ADI (France) 0.0025 mg/kg (provisional). Toxicity class WHO (a.i.) U
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Fish Low acute toxicity to rainbow trout; LC50 >10 mg/l. Daphnia LC50 (48 h) >9.3 mg/l. Algae EC50 (96 h) >1.0 mg/l. Worms Non-toxic.
ENVIRONMENTAL FATE
Animals 90% Elimination via faeces within 7 days in the rat. Plants Metabolised to a dihydroxy metabolite and others. Soil/Environment DT50 224-360 d (10 ºC).
|