|
trichlorfon
Insecticide
IRAC 1B; organophosphate
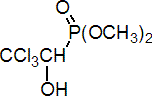
NOMENCLATURE
Common name trichlorfon (BSI (since 1984), E-ISO, (m) F-ISO); chlorophos (USSR); metriphonate (BAN); DEP (JMAF); trichlorphon* (BSI (before 1984)); dipterex* (former exception, Turkey)
IUPAC name dimethyl 2,2,2-trichloro-1-hydroxyethylphosphonate
Chemical Abstracts name dimethyl (2,2,2-trichloro-1-hydroxyethyl)phosphonate
Other names metrifonate CAS RN [52-68-6] EEC no. 200-149-3 Development codes Bayer 15 922; Bayer L13/59 Official codes OMS 800; ENT 19 763
PHYSICAL CHEMISTRY
Composition Racemate, i.e. 1:1 mixture of the (1R)- and (1S)- enantiomers. Mol. wt. 257.4 M.f. C4H8Cl3O4P Form Colourless crystals, with a weak, characteristic odour. M.p. 78.5 ºC; delayed melting to 84 ºC V.p. 0.21 mPa (20 ºC); 0.5 mPa (25 ºC) KOW logP = 0.43 (20 °C) Henry 4.4 ´ 10-7 Pa m3 mol-1 (20 °C) S.g./density 1.73 (20 ºC) Solubility In water 120 g/l (20 ºC). Readily soluble in common organic solvents (with the exception of aliphatic hydrocarbons and petroleum oils), e.g. hexane 0.1-1, dichloromethane, isopropanol >200, toluene 20-50 (all in g/l, 20 ºC). Stability Subject to hydrolysis and dehydrochlorination. Decomposition proceeds more rapidly with heating, and above pH 6. Rapidly converted by alkalis to dichlorvos (q.v.), which is then hydrolysed: DT50 (22 ºC) 510 d (pH 4), 46 h (pH 7), <30 min (pH 9). Photolysis is slow.
COMMERCIALISATION
History Insecticide reported by G. Unterstenhöfer (Anz. Schaedlingskd., 1957, 30, 7). First prepared by W. Lorenz, and introduced by Bayer AG in 1957. Patents US 2701225 Manufacturers Bayer CropScience; Cequisa; Denka; Jin Hung; Lucava; Makhteshim-Agan; Sanonda
APPLICATIONS
Biochemistry Cholinesterase inhibitor; activity may be due to in vivo conversion to dichlorvos (q.v.). Mode of action Non-systemic insecticide with contact and stomach action. Uses Insecticidal control in agriculture, horticulture, forestry, food storage, gardening, households, and animal husbandry. In particular, control of Diptera, Lepidoptera, Hymenoptera, Hemiptera, and Coleoptera on many crops. For agricultural uses, applied at 300-1200 g/ha. Also used to control household pests such as flies, cockroaches, fleas, bed bugs, silverfish, ants, etc.; as a fly bait in farm buildings and animal houses; and for control of ectoparasites on domestic animals. Formulation types DP; GB; GR; PO; SC; SL; SP; UL; WP; Coating agent. Compatibility Incompatible with alkaline materials and oils. Selected products: 'Cekufon' (Cequisa); 'Danex' (Makhteshim-Agan); 'Denkaphon' (Denka); 'Dipterex' (Bayer CropScience); 'Lucavex' (Lucava); 'Saprofon' (Sanonda)
OTHER PRODUCTS
'Dylox' (Bayer CropScience); 'Neguvon' (Bayer CropScience) Discontinued products: 'Briten' * (Q.E.A.C.A.); 'Ditrifon' * (Chemolimpex)
ANALYSIS
Product analysis by polarography (P. A. Giang & R. L. Caswell, J. Agric. Food Chem., 1957, 5, 753) or by titration of the chloride ion obtained by hydrolysis (CIPAC Handbook, 1970, 1, 684; FAO Specification (CP/51)). Residues determined by glc (Man. Pestic. Residue Anal., 1987, I, 6, S13, S19; Anal. Methods Residues Pestic., 1988, Part I, M2, M5, M12; A. Ambrus et al., J. Assoc. Off. Anal. Chem., 1981,64, 733). Details available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Reviews JECFA 54, FAO/WHO 30, 31 (see part 2 of the Bibliography). IARC ref. 30 class 3 Oral Acute oral LD50 for male and female rats c. 250 mg/kg. Skin and eye Acute percutaneous LD50 (24 h) for male and female rats >5000 mg/kg. Not irritating to skin and eye (rabbits). Inhalation LC50 (4 h) for male and female rats >2.3 mg/l air (aerosol). NOEL (2 y) for rats 100, for mice 300 mg/kg diet; (4 y) for dogs 50 mg/kg diet. ADI (JECFA) 0.02 mg/kg b.w. [2000]; (JMPR) 0.01 mg/kg b.w. [1978]. Toxicity class WHO (a.i.) II; EPA (formulation) II EC classification Xn; R22| R43
ECOTOXICOLOGY
Fish LC50 (96 h) for rainbow trout 0.7, golden orfe 0.52 mg/l. Daphnia LC50 (48 h) 0.00096 mg/l. Algae ErC50 for Scenedesmus subspicatus >10 mg/l. Bees Low toxicity to bees and other beneficial insects.
ENVIRONMENTAL FATE
EHC 132 (WHO, 1992); 63 (WHO, 1986; a general review of organophosphorus insecticides). EHC 132 notes that trichlorfon is moderately toxic to fish and birds, and moderately to highly toxic to aquatic arthropods, and concludes it should not be sprayed over areas of water. Animals In animals, trichlorfon is rapidly absorbed and metabolised. Excretion of the radioactivity in the urine is more or less complete within 6 hours. The major metabolites are dimethylphosphoric acid, monomethylphosphoric acid and conjugates of dichloroacetic acid. Plants In plants, trichlorfon is rapidly hydrolysed. The main metabolites are dimethyl- and monomethyl- phosphoric acid and conjugates of dichloroacetic acid and dichloroethanol. Soil/Environment Trichlorfon has a relatively high mobility in soil, but it is rapidly metabolised to CO2. Intermediates are dichloroethanol, dichloroacetic acid and trichloroacetic acid. Koc 20 (?0).
|