|
triadimenol
Fungicide
FRAC 3, G1; DMI: triazole
NOMENCLATURE
Common name triadimenol (BSI, E-ISO, (m) F-ISO)
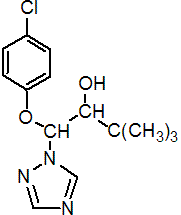
IUPAC name (1RS,2RS;1RS,2SR)-1-(4-chlorophenoxy)-3,3-dimethyl-1-(1H-1,2,4-triazol-1-yl)butan-2-ol
Chemical Abstracts name b-(4-chlorophenoxy)-a-(1,1-dimethylethyl)-1H-1,2,4-triazole-1-ethanol
CAS RN [55219-65-3] unstated stereochemistry; [89482-17-7] diastereoisomer A; [82200-72-4] diastereoisomer B EEC no. 259-537-6 Development codes BAY KWG 0519 (Bayer)
PHYSICAL CHEMISTRY
Composition (1RS,2SR) is referred to here as diastereoisomer A; (1RS,2RS) is referred to here as diastereoisomer B. The ratio of A:B is c. 7:3. Mol. wt. 295.8 M.f. C14H18ClN3O2 Form Colourless odourless crystals. M.p. A: 138.2 ºC; B: 133.5 ºC; eutectic A+B: 110 ºC; (tech., 103-120 ºC) V.p. A: 6 ´ 10-4 mPa; B: 4 ´ 10-4 mPa (20 °C) KOW A: logP = 3.08; B: logP = 3.28 (25 °C) Henry A: 3 ´ 10-6; B: 4 ´ 10-6 (Pa m3 mol-1, 20 °C) S.g./density A: 1.237; B: 1.299 (22 °C) Solubility In water A: 62; B: 33 mg/l (20 ºC). In dichloromethane >250, isopropanol 50-100, hexane 0.1-1.0, n-heptane 0.45, xylene 18, toluene 20-50 (all in g/l, 20 °C). Stability Both diastereoisomers are stable to hydrolysis; DT50 (20 ºC) >1 y (pH 4, 7 or 9).
COMMERCIALISATION
History Fungicide reported by P. E. Frohberger (Pflanzenschutz-Nachr. (Engl. Ed.), 1978, 31, 11). Introduced by Bayer AG and first marketed in 1978. Patents DE 2324010 Manufacturers Bayer CropScience; Makhteshim-Agan; Sannong; Sharda
APPLICATIONS
Biochemistry Inhibits gibberellin and ergosterol biosynthesis and hence the rate of cell division. Mode of action Systemic fungicide with protective, curative and eradicant action. Absorbed by the roots and leaves, with ready translocation in young growing tissues, but less ready translocation in older, woody tissues. Uses Control of powdery mildews, rusts and Rhynchosporium in cereals, and, when applied as a seed treatment, control of bunt, smuts, Typhula spp., seedling blight, leaf stripe, net blotch and other cereal diseases. Also used on vegetables, ornamentals, coffee, hops, vines, fruit, tobacco, sugar cane, bananas and other crops, mainly against powdery mildews, rusts and various leaf spot diseases. Application rates as a spray are in the range 100-250 g/ha for bananas and cereals, 125-250 g/ha protective and 250-500 g/ha eradicative for coffee, 0.0025-0.0125% for grapes, pome and stone fruit and vegetables; application rates as a seed treatment are in the range 20-60 g/100 kg seed for cereals, 30-60 g/100 kg seed for cotton. Formulation types DP; DS; EC; EW; FS; GR; SC; WG; WP; WS. Selected products: 'Bayfidan' (spray) (Bayer CropScience); 'Euro' (Rocca); 'Noidio' (Agrimix); 'Shavit' (Makhteshim-Agan); 'Vydan' (Vapco); mixtures: 'Baytan C' (+ fuberidazole+ imidacloprid) (seed treatment) (Bayer CropScience); 'Baytan Combi' (+ fuberidazole+ imazalil) (fuberidazole not present in some formulations) (Bayer CropScience); 'Baytan Secur' (+ fuberidazole+ imidacloprid) (seed treatment) (Bayer CropScience); 'Baytan Spezial' (+ fuberidazole) (seed treatment) (Bayer CropScience); 'Baytan Universal' (+ fuberidazole+ imazalil) (seed treatment) (Bayer CropScience); 'Horizon' (+ tebuconazole) (spray) (Bayer CropScience); 'Matador' (+ tebuconazole) (spray) (Bayer CropScience)
OTHER PRODUCTS
'Atrizan' (with superphosphate) (Bayer CropScience); 'Baytan FS' (Bayer CropScience); 'Prodimenol' (Probelte) mixtures: 'Abilis' (+ tebuconazole) (spray, France) (Bayer CropScience); 'Bayfidan BC' (+ carbendazim) (Bayer CropScience); 'Bayfidan Blau' (+ propineb) (Bayer CropScience); 'Bayfidan BM' (+ carbendazim) (Bayer CropScience); 'Bayfidan S' (+ sulfur) (Bayer CropScience); 'Bayfidan Triple' (+ disulfoton+ fenamiphos) (Bayer CropScience); 'Baysiston' (+ disulfoton) (Bayer CropScience); 'Baytan IM' (+ fuberidazole+ imazalil) (Bayer CropScience); 'Baytan T' (+ cypermethrin) (seed treatment) (Bayer CropScience); 'Cereline Secur' (+ bitertanol+ fuberidazole+ imidacloprid) (UK) (Bayer CropScience); 'Cereline Secur' (+ fuberidazole+ imidacloprid) (seed treatment) (Bayer CropScience); 'Falcon' (+ spiroxamine+ tebuconazole) (spray) (Bayer CropScience); 'Garnet' (+ tebuconazole) (spray, UK) (Bayer CropScience); 'Manta Plus' (+ fuberidazole+ imazalil+ imidacloprid) (Germany) (Bayer CropScience); 'Repulse' (+ disulfoton) (Bayer CropScience); 'Shavit F' (+ folpet) (Makhteshim-Agan); 'Silvacur Combi' (+ tebuconazole) (spray, Central America) (Bayer CropScience); 'Silvacur' (+ tebuconazole) (spray, UK) (Bayer CropScience); 'Veto F' (+ tebuconazole) (spray, UK) (Bayer CropScience) Discontinued products: 'Spinnaker' * (Cyanamid) mixtures: 'Baytan TA' * (+ triazoxide+ anthraquinone) (France) (Bayer); 'Brio Flo' * (+ triazoxide+ anthraquinone) (France) (Bayer); 'Brio' * (+ triazoxide+ anthraquinone) (France) (Bayer); 'Cereline' * (+ bitertanol+ fuberidazole) (Bayer); 'Colt' * (+ tridemorph) (Bayer); 'Domestin' * (+ bitertanol+ fuberidazole) (Bayer); 'Dorin' * (+ tridemorph) (Bayer); 'Folicur Bayfidan' * (+ tebuconazole) (spray, Switzerland) (Bayer)
ANALYSIS
Product analysis by glc with FID (CIPAC Handbook, 1992, E, 224-229). Residues determined by glc (W. Specht & M. Tillkes, Pflanzenschutz-Nachr. (Engl. Ed.), 1980, 33, 61; R. Brennecke, ibid., 1984, 37, 68; A. Allmendinger, ibid., 1991, 44, 5). Methods for the determination of residues are available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 56, 58 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats c. 700, mice c. 1300 mg/kg. Skin and eye Acute percutaneous LD50 for rats >5000 mg/kg. Non-irritating to eyes and skin (rabbits). Not a skin sensitiser. Inhalation LC50 (4 h) for rats >0.95 mg/l air (aerosol). NOEL (2 y) for rats 125, for dogs 600, male mice 80, female mice 400 mg/kg diet. ADI (JMPR) 0.05 mg/kg b.w. [1989]. Other No mutagenic or teratogenic effects. Toxicity class WHO (a.i.) III; EPA (formulation) III EC classification (Xn; R22)
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Fish LC50 (96 h) for golden orfe 17.4, rainbow trout 21.3, bluegill sunfish 15 mg/l. Daphnia LC50 (48 h) 51 mg/l. Algae ErC50 for Scenedesmus subspicatus 3.7 mg/l. Bees Non-toxic to honeybees. Worms LC50 for Eisenia foetida 772 mg/kg dry soil.
ENVIRONMENTAL FATE
Animals In rats, triadimenol was metabolised mainly by oxidation of the tert-butyl moiety to the corresponding alcohol and then to carboxylic acid. A small fraction of these compounds was conjugated. Oxidation at the hydroxyl group to the corresponding keto compound (triadimefon, q.v.) and its subsequent oxidation at the tert-butyl moiety was of secondary importance. Plants The most important breakdown reactions of triadimenol in plants after seed treatment and spray application are conjugation of the a.i. with various sugar compounds (especially hexose) and oxidation at the tert-butyl moiety. The resulting primary alcohol is likewise partly conjugated. After seed treatment, the 1,2,4-triazole formed in the soil by hydrolysis was taken up into the plant via the roots and conjugated with various endogenous plant substances. Soil/Environment In soil, triadimenol is a degradation product of triadimefon (q.v.) and degradation depends on microbial activity. The first step is the oxidation of t-butyl moiety, followed by rapid degradation steps like the loss of t-butyl moiety and cleavage of the triazole ring; degradation involving hydrolytic cleavage leads to the formation of 4-chlorophenol. Metabolism of the individual triadimenol enantiomers proceeds at different rates (T. Clark et al., Proc. Br. Crop Prot. Conf. - Pests Dis., 1986, 475). DT50 in sandy loam 110-375 d; in loam 240-270 d.
|