|
transfluthrin
Insecticide
IRAC 3; pyrethroid
NOMENCLATURE
Common name transfluthrin (BSI, pa E-ISO)
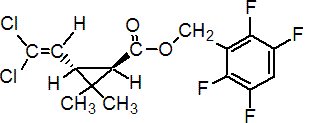
IUPAC name 2,3,5,6-tetrafluorobenzyl (1R,3S)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Roth: 2,3,5,6-tetrafluorobenzyl (1R)-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Chemical Abstracts name (1R-trans)-(2,3,5,6-tetrafluorophenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
Other names benfluthrin* (rejected BSI name proposal) CAS RN [118712-89-3] EEC no. 405-060-5 Development codes NAK 4455
PHYSICAL CHEMISTRY
Composition Tech. is ³92%. Mol. wt. 371.2 M.f. C15H12Cl2F4O2 Form Colourless crystals. M.p. 32 ºC B.p. 135 ºC/0.1 mbar V.p. 4.0 ´ 10-1 mPa (20 ºC) KOW logP = 5.46 (20 ºC) Henry 2.60 Pa m3 mol-1 (calc.) S.g./density 1.5072 g/cm3 (23 ºC) Solubility In water 5.7 ´ 10-5 g/l (20 ºC). In organic solvents >200 g/l. Stability No decomposition after 5 h at 200 ºC. DT50 in pure water (25 ºC) >1 y (pH 5), >1 y (pH 7), 14 d (pH 9). Specific rotation [a]D29 +15.3?(c=0.5, CHCl3)
COMMERCIALISATION
Patents DE 2714042; DE 3705224 both to Bayer Manufacturers Bayer CropScience; Jiangsu Yangnong
APPLICATIONS
Biochemistry Effector on presynaptic voltage gate sodium channels in nerve membranes, causing knockdown effects in insects. Mode of action Insecticide active by inhalation and contact; also repellent. Uses Fast-acting insecticide against mosquitoes, flies, cockroaches and whitefly. Formulation types AL; FU; LV; MC; VP; XX; Spraying cans. Selected products: 'Baygon' (Bayer CropScience); 'Bayothrin' (Bayer CropScience)
ANALYSIS
Methods for the determination of residues are available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male and female rats >5000, male mice 583, female mice 688, hens >5000 mg/kg. Skin and eye Acute percutaneous LD50 (24 h) for male and female rats >5000 mg/kg. Inhalation LC50 (4 h) for male and female rats >513 mg/m3 air. NOEL (24 mo) for male and female rats 20 ppm, for male and female mice 100 ppm. Toxicity class WHO (a.i.) U EC classification Xi; R38| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for Virginian tree quail (Colinus virginianus) and canary birds (Serinus canarius) >2000 mg/kg. Fish LC50 (96 h) for golden orfe 1.25, rainbow trout 0.7 mg/l. Daphnia LC50 (48 h) 0.0017 mg/l. Algae EC50 (96 h) for Scenedesmus subspicatus >0.1 mg/l.
ENVIRONMENTAL FATE
Animals Tetrafluorobenzoic acid and the glucuronate of tetrafluorobenzyl alcohol are formed. Soil/Environment In aquatic ecosystems, tetrafluorobenzyl alcohol and tetrafluorobenzoic acid are formed. DT50 in water 2 d (in light), 8 d (in dark).
|