|
tralomethrin
Insecticide
IRAC 3; pyrethroid
NOMENCLATURE
Common name tralomethrin (BSI, ANSI, draft E-ISO); tralométhrine (draft F-ISO)
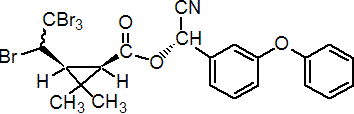
IUPAC name (S)-a-cyano-3-phenoxybenzyl (1R,3S)-2,2-dimethyl-3-[(RS)-1,2,2,2-tetrabromoethyl]cyclopropanecarboxylate
Roth: (S)-a-cyano-3-phenoxybenzyl (1R)-cis-2,2-dimethyl-3-[(RS)-1,2,2,2-tetrabromoethyl]cyclopropanecarboxylate
Chemical Abstracts name cyano(3-phenoxyphenyl)methyl 2,2-dimethyl-3-(1,2,2,2-tetrabromoethyl)cyclopropanecarboxylate
CAS RN [66841-25-6] unstated stereochemistry Development codes NU 831; RU 25474 (Roussel Uclaf); HAG 107 Official codes OMS 3048
PHYSICAL CHEMISTRY
Composition Laboratory grade tralomethrin (>93% a.i.) is a 60:40 mixture of two active diastereoisomers. Mol. wt. 665.0 M.f. C22H19Br4NO3 Form Yellow to beige, resinous solid (tech.). M.p. 138-148 ºC V.p. 4.8 ´ 10-6 mPa (25 °C) KOW logP = c. 5 (25 °C) S.g./density 1.70 (20 ºC) Solubility In water 80 mg/l. In acetone, dichloromethane, toluene, xylene >1000, dimethyl sulfoxide >500, ethanol >180 (all in g/l). Stability Stable for 6 months at 50 ºC. Acidic media reduce hydrolysis and epimerisation. Specific rotation [a]D +21?to +27?(50 g/l toluene) F.p. 26 ºC
COMMERCIALISATION
History Discovered and introduced by Roussel Uclaf (now Bayer CropScience). Patents FR 2364884 Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Activity may be due to debromination to form deltamethrin (q.v.). Mode of action Non-systemic insecticide with contact and stomach action. Uses Insecticide with ingested and contact action. Controls a range of agronomic pests (Coleoptera, Homoptera, Orthoptera and particularly Lepidoptera, in cereals, coffee, cotton, fruit, maize, oilseed rape, rice, tobacco and vegetables), at 7.5-20 g/ha. If applied before infestations are well established, protects most crops against plant-sucking Hemiptera. Soil-surface sprays (5-10 g/ha) control cutworms (Agrotis and Euxoa spp.). Effective for wood protection, insect household pests, in public health, stored grains (Blattodea, Culicidae, Muscidae, grain borers and wood-attacking insects). Also very effective on Muscidae, Tabanidae, Ixodidae and other Acari on cattle, sheep and pigs, by application as dip, spray or pour-on. Formulation types EC; SC; WP. Selected products: 'Saga' (environmental health uses) (Bayer CropScience); 'Scout' (crop protection uses) (Bayer CropScience); 'Tralox' (veterinary use) (Intervet)
OTHER PRODUCTS
'Scout-Xtra' (Bayer CropScience); 'Stryker' (Bayer CropScience); 'Tracker' (DuPont); 'Tralate' (DuPont) Discontinued products mixtures: 'Synergin 1+2' * (+ amitraz) (Aventis)
ANALYSIS
Product analysis by hplc. Residues determined by glc with ECD.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 99-3000 mg a.i./kg, depending on the carrier used; for dogs >500 mg (in capsules)/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Moderate skin irritant; mild eye irritant (rabbits). Inhalation LC50 (4 h) for rats >0.40 mg/l air. NOEL (2 y) for rats 0.75 mg/kg b.w. daily; for mice 3 mg/kg b.w. daily; for dogs 1 mg/kg b.w. daily. ADI 0.0075 mg/kg b.w. Other Tech. not mutagenic or teratogenic in rats or rabbits. Toxicity class WHO (a.i.) II; EPA (formulation) III
ECOTOXICOLOGY
Birds Acute oral LD50 for quail >2510 mg/kg. Dietary LC50 (8 d) for mallard ducks 7716, quail 4300 mg/kg diet. Fish LC50 (96 h) for rainbow trout 0.0016, bluegill sunfish 0.0043 mg/l. Daphnia LC50 (48 h) 38 ng/l. Low LC50 and LD50 values under laboratory conditions do not represent significant hazard to aquatic fauna in normal field use. Bees Non-toxic to honeybees. LD50 (contact) 0.12 mg/bee. Low LC50 and LD50 values under laboratory conditions do not represent significant hazard to bees in normal field use.
ENVIRONMENTAL FATE
Animals Tralomethrin is transformed into deltamethrin and further degraded. Plants Tralomethrin is transformed into deltamethrin. Soil/Environment Strongly adsorbed in soil; DT50 64-84 d. Kd 197-8784; Koc 43 796-675 667; highly immobile in various soils (sandy to clay loam).
|