|
thiocyclam
Insecticide
IRAC 4C; 2-dimethylaminopropane-1,3-dithiol analogue
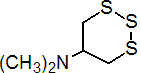
NOMENCLATURE
thiocyclam
Common name thiocyclam (BSI, E-ISO, JMAF); thiocyclame ((m) F-ISO)
CAS RN [31895-21-3] Development codes SAN 155I
thiocyclam hydrogen oxalate
IUPAC name N,N-dimethyl-1,2,3-trithian-5-ylamine hydrogen oxalate
Chemical Abstracts name N,N-dimethyl-1,2,3-trithian-5-amine hydrogen oxalate
CAS RN [31895-22-4] EEC no. 250-859-2 Development codes SAN 155I
PHYSICAL CHEMISTRY
thiocyclam
Mol. wt. 181.3 M.f. C5H11NS3
thiocyclam hydrogen oxalate
Mol. wt. 271.4 M.f. C7H13NO4S3 Form Colourless, odourless solid. M.p. 125-128 ºC (decomp.) V.p. 0.545 mPa (20 ºC) KOW logP = -0.07 (unstated pH) Henry 1.8 ´ 10-6 Pa m3 mol-1 (calc.) S.g./density 0.6 Solubility In water 84 (pH <3.3, 23 ºC), 44.1 (pH 3.6, 20 ºC), 16.3 (pH 6.8, 20 ºC) (all in g/l). In dimethyl sulfoxide 92, methanol 17, ethanol 1.9, acetonitrile 1.2, acetone 0.5, ethyl acetate, chloroform <1, toluene, hexane <0.01 (all in g/l, 23 ºC). Stability Stable during storage, shelf life (20 ºC) ³2 y; degraded by sunlight, DT50 2-3 d in surface waters; hydrolysis DT50 (25 ºC) 0.5 y (pH 5), 5-7 d (pH 7-9). pKa pKa1 3.95, pKa2 7.00
COMMERCIALISATION
History Insecticide reported by W. Berg & H. J. Knutti (Proc. Br. Insectic. Fungic. Conf. 8th, 1975, 2, 683). Its hydrogen oxalate was introduced by Sandoz Agro AG (later Syngenta AG, who sold rights to Arysta LifeScience Corp. and Nippon Kayaku Co., Ltd in 2002). Patents DE 2039555 Manufacturers Nippon Kayaku; Sundat
APPLICATIONS
Biochemistry Analogue or propesticide of the natural toxin nereistoxin. Nicotinergic acetylcholine blocker, causing paralysis by ganglionic blocking action on the central nervous system. Mode of action Selective insecticide with contact and stomach action. Limited systemic activity, with translocation acropetally.
thiocyclam hydrogen oxalate
Uses Control of Lepidoptera, Coleoptera, some Diptera and Thysanoptera. In potatoes for Colorado potato beetle, in rape for Coleoptera and Lepidoptera pest complexes, in irrigated rice for stem borers and some other pests, in maize for corn borer and Tanymecus, in sugar beet for sugar beet weevil and other Coleoptera, in sugar cane for sugar cane stem borer, in fruit trees for Lepidoptera, in vegetables for leaf miners, and various Lepidoptera and Coleoptera. Phytotoxicity Non-phytotoxic at recommended dose to most crops, except for Stark varieties of apple. Formulation types DP; WP; SP; GR; Dust. Compatibility Incompatible with copper-containing fungicides. Selected products: 'Evisect' (Nippon Kayaku, Arysta)
ANALYSIS
Product analysis by hplc (M. Wisson et al., Anal. Methods Pestic. Plant Growth Regul., 1980, 11, 185). Residues determined by glc with FPD or ECD (idem, ibid., p. 190).
MAMMALIAN TOXICOLOGY
thiocyclam
Toxicity class WHO (a.i.) II
thiocyclam hydrogen oxalate
Oral Acute oral LD50 for male rats 399, female rats 370, male mice 273 mg/kg. Skin and eye Acute percutaneous LD50 for male rats 1000, female rats 880 mg/kg. Non-irritant to skin and eyes. Inhalation LC50 (1 h) for rats >4.5 mg/l air. NOEL (2 y) for rats 100 mg/kg diet, for dogs 75 mg/kg diet. EC classification Xn; R21/22| N; R50, R53
ECOTOXICOLOGY
thiocyclam hydrogen oxalate
Birds Acute oral LD50 for quail 3.45 mg/kg. Dietary LC50 (8 d) for quail 340 mg/kg diet. Fish LC50 (96 h) for carp 1.01, trout 0.04 mg/l. Daphnia LC50 (48 h) 2.01 mg/l. Algae EC50 (96 h) for green algae 3.3 mg/l. Bees Moderately toxic to bees; LD50 (24 h, oral) 11.9 mg a.i./bee ('Evisect S').
ENVIRONMENTAL FATE
Plants Degradation in plants is the same as in soil. Soil/Environment Thiocyclam hydrogen oxalate, via nereistoxin and its oxide, is broken down to smaller fragments. Degradation is accelerated by light. DT50 in soil 1 d (o.c. 2.8%, pH 6.8, 22 ºC).
|