|
thiamethoxam
Insecticide
IRAC 4A; neonicotinoid
NOMENCLATURE
Common name thiamethoxam (BSI, pa ISO)
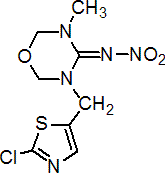
IUPAC name 3-(2-chloro-1,3-thiazol-5-ylmethyl)-5-methyl-1,3,5-oxadiazinan-4-ylidene(nitro)amine
Chemical Abstracts name 3-[(2-chloro-5-thiazolyl)methyl]tetrahydro-5-methyl-N-nitro-4H-1,3,5-oxadiazin-4-imine
CAS RN [153719-23-4] Development codes CGA 293343 (Ciba-Geigy)
PHYSICAL CHEMISTRY
Mol. wt. 291.7 M.f. C8H10ClN5O3S Form Crystalline powder. M.p. 139.1 °C V.p. 6.6 ´ 10-6 mPa (25 °C) KOW logP = -0.13 (25 °C) Henry 4.70 ´ 10-10 Pa m3 mol-1 (calc.) Solubility In water 4.1 g/l (25 °C).
COMMERCIALISATION
History Discovered by Ciba (now Syngenta AG) in 1991. Reported by R. Senn et al. (Proc. Br. Crop Prot. Conf. - Pests Dis., 1998, 1, 27). Introduced in New Zealand in 1997. Patents EP 580553 Manufacturers Syngenta
APPLICATIONS
Biochemistry Agonist of the nicotinic acetylcholine receptor, affecting the synapses in the insect central nervous system. Mode of action Insecticide with contact, stomach and systemic activity. Rapidly taken up into the plant and transported acropetally in the xylem. Uses For control of aphids, whitefly, thrips, ricehoppers, ricebugs, mealybugs, white grubs, Colorado potato beetle, flea beetles, wireworms, ground beetles, leaf miners and some lepidopterous species, at application rates from 10 to 200 g/ha (R. Senn et al., loc. cit.). Major crops for foliar and soil treatments are cole crops, leafy and fruity vegetables, potatoes, rice, cotton, deciduous fruit, citrus, tobacco and soya beans; for seed treatment use, maize, sorghum, cereals, sugar beet, oilseed rape, cotton, peas, beans, sunflowers, rice and potatoes. Also for control of flies in animal and public health, such as Musca domestica, Fannia canicularis, and Drosophila spp. Formulation types FS; GR; SC; WG; WS. Selected products: 'Actara' (foliar and soil treatments) (Syngenta); 'Cruiser' (seed treatment) (Syngenta); 'Agita' (animal and public health) (Novartis A H)
OTHER PRODUCTS
'Adage' (USA) (Syngenta); 'Centric' (Syngenta); 'Flagship' (Syngenta); 'Meridian' (Syngenta); 'Platinum' (USA) (Syngenta) mixtures: 'Helix' (+ difenoconazole+ fludioxonil+ metalaxyl-M) (Syngenta)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 1563 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin and eyes (rabbits); not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >3720 mg/m3. Toxicity class WHO (a.i.) III (company classification)
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 1552, mallard ducks 576 mg/kg. Dietary LC50 for bobwhite quail and mallard ducks >5200 mg/kg. Fish LC50 (96 h) for rainbow trout >100, bluegill sunfish >114, sheepshead minnow >111 mg/l. Daphnia EC50 (48 h) >100 mg/l. Algae EC50 (96 h) for green algae >100 mg/l. Other aquatic spp. LC50 (96 h) for mysid shrimp 6.9 mg/l; EC50 (96 h) for Eastern oyster >119 mg/l. Bees LD50 for honeybees (contact) 0.024 mg/bee. Worms LC50 (14 d) for Eisenia foetida >1000 mg/kg soil.
ENVIRONMENTAL FATE
Animals Quickly and completely absorbed, rapidly distributed in the body and rapidly eliminated. The toxicokinetics and metabolism are not influenced by the route of administration, the dose level, pre-treatment, the site of label or the sex of animals. The major metabolic pathways are essentially the same in rats as in mice, goats and hens. Plants Degradation/metabolism has been studied in 6 different crops with soil, foliar and seed treatment application. The qualitative metabolic pattern was similar for all types of applications and for all studied crops. Soil/Environment Soil DT50 (median) 51 d. Stable in water under acid conditions, hydrolysed under alkaline conditions. Aqueous photolysis occurs rapidly. No bioaccumulation.
|