|
tetraconazole
Fungicide
FRAC 3, G1; DMI: triazole
NOMENCLATURE
Common name tetraconazole (BSI, draft E-ISO)
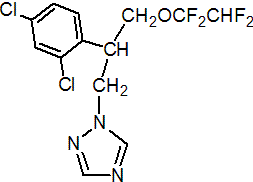
IUPAC name (RS)-2-(2,4-dichlorophenyl)-3-(1H-1,2,4-triazol-1-yl)propyl 1,1,2,2-tetrafluoroethyl ether
Chemical Abstracts name (?-1-[2-(2,4-dichlorophenyl)-3-(1,1,2,2-tetrafluoroethoxy)propyl]-1H-1,2,4-triazole
CAS RN [112281-77-3] unstated stereochemistry EEC no. 407-706-6 Development codes M 14360 (Agrimont)
PHYSICAL CHEMISTRY
Mol. wt. 372.1 M.f. C13H11Cl2F4N3O Form Colourless, viscous liquid; (tech., yellow to yellowish-brown liquid). M.p. Pour point 6 °C B.p. Decomp. 240 ºC without boiling V.p. 0.18 mPa (gas saturation method) KOW logP = 3.56 (20 ºC) Henry 3.6 ´ 10-4 Pa m3 mol-1 (20 °C, calc.) S.g./density 1.432 (20 °C) Solubility In water 156 mg/l (pH 7, 20 ºC). Readily soluble in 1,2-dichloroethane, acetone, and methanol. Stability Stable in dilute aqueous solutions at pH 4-9. Stable in water to sunlight.
COMMERCIALISATION
History Fungicide reported by C. Garavaglia et al. (Proc. 1988 Br. Crop Prot. Conf. - Pests Dis., 1, 49). Introduced by Agrimont S.p.A. (now Isagro S.p.A.). Patents EP 234242 Manufacturers Isagro
APPLICATIONS
Biochemistry Sterol C14-demethylase inhibitor. Mode of action Broad-spectrum systemic fungicide with protective, curative and eradicant properties. Absorbed by the roots, stem and leaves, with translocation acropetally to all parts of the plant, including subsequent growth. Uses Control of powdery mildew, brown rust, Septoria and Rhynchosporium on cereals; powdery mildew and scab on pome fruit; powdery mildew on vines and cucumbers; powdery mildew and beet leaf spot on sugar beet; and powdery mildew and rust on vegetables and ornamentals. Applied at 2.5-5 g/hl for fruit and vegetables, 100-125 g/ha for field crops. Formulation types EC; ME; LS; SE. Selected products: 'Domark' (Isagro); 'Eminent' (Isagro); 'Lospel' (Isagro)
OTHER PRODUCTS
'Arpège' (Sipcam Phyteurop); 'Arum' (Bayer CropScience); 'Buongiorno' (Arysta, Isagro); 'Defender' (Sipcam); 'Fief' (Bayer CropScience); 'Greman' (Sipcam Phyteurop); 'Hokuguard' (Arysta, Isagro); 'Juggler' (Sipcam UK); 'Timbal' (Sipcam Phyteurop) mixtures: 'Eminent Star' (+ chlorothalonil) (Isagro); 'Abitre' (+ chlorothalonil) (Bayer CropScience); 'Arbitre' (+ chlorothalonil) (Bayer CropScience); 'Bonanza' (+ prochloraz) (Sipcam Phyteurop); 'Music' (+ chlorothalonil) (Sipcam Phyteurop); 'Timbal F' (+ fentin hydroxide) (Sipcam Phyteurop); 'Voodoo' (+ chlorothalonil) (UK) (Sipcam) Discontinued products mixtures: 'Voodoo' * (+ chlorothalonil) (Monsanto)
ANALYSIS
Product and residue analysis by glc or hplc. Details available from Isagro.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 1248, female rats 1031 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Not irritating to skin; slightly irritating to eyes (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >3.66 mg/l. NOEL In 2 y study, NOAEL for rats 80 mg/kg diet. Other Non-mutagenic. Toxicity class WHO (a.i.) II EC classification R40| Xn; R20/22| N; R51, R53
ECOTOXICOLOGY
Birds Dietary LC50 (5 d) for bobwhite quail 650, mallard ducks 422 mg/kg. Fish LC50 (96 h) for rainbow trout 4.8, bluegill sunfish 4.3 mg/l. Daphnia LC50 (48 h) 3.0 mg/l. Bees LD50 (oral) >130 mg/bee.
ENVIRONMENTAL FATE
Animals Tetraconazole is readily adsorbed, metabolised and excreted with no significant retention in the tissues. The main metabolite identified in urine of rats is 1,2,4-triazole. Plants Extensive metabolism occurs in plants. The identified metabolites are tetraconazole acid, tetraconazole alcohol, triazolylalanine and triazolylacetic acid. Soil/Environment No accumulation in the field. No leaching occurs in standard soils. Koc 531-1922 in 4 soil types.
|