|
tepraloxydim
Herbicide
HRAC A WSSA 1; cyclohexanedione oxime
NOMENCLATURE
Common name tepraloxydim (BSI, pa ISO)
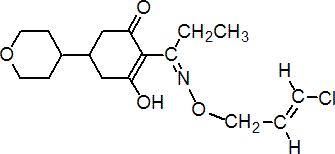
IUPAC name (EZ)-(RS)-2-{1-[(2E)-3-chloroallyloxyimino]propyl}-3-hydroxy-5-perhydropyran-4-ylcyclohex-2-en-1-one
Chemical Abstracts name (E,?)-2-[1-[[(3-chloro-2-propenyl)oxy]imino]propyl]-3-hydroxy-5-(tetrahydro-2H-pyran-4-yl)-2-cyclohexen-1-one
Other names caloxydim* (BSI rejected name proposal) CAS RN [149979-41-9] Development codes BAS 620H (BASF)
PHYSICAL CHEMISTRY
Mol. wt. 341.8 M.f. C17H24ClNO4 Form White, odourless powder. M.p. 74 °C V.p. 1.1 ´ 10-2 mPa (20 °C) Solubility In pure water 0.43 g/l (20 °C). pKa 4.58 (20 °C)
COMMERCIALISATION
History A herbicide of Nisso BASF Agro Ltd, a joint venture company of Nippon Soda Co., Ltd, BASF AG and Mitsui Corporation Inc. Under development in Europe and the Americas by BASF and first registered in 1999. Reported by E. Kibler et al., Proc. Br. Crop Prot. Conf. -Weeds, 1999, 1, 59. Discovered by BASF. Manufacturers Nisso BASF Agro
APPLICATIONS
Biochemistry Fatty acid synthesis inhibitor, by inhibition of acetyl CoA carboxylase (ACCase). Mode of action Absorbed through the leaves and translocated throughout the plant to the roots. Becomes rainfast within 1 h. Treated grasses cease growth, followed by leaf tip necrosis, reddening of foliage and subsequent die-back. Uses Under development for broad-spectrum post-emergence grass weed control, especially Poa annua and volunteer maize, and also Sorghum halepense, Elymus repens, in broad-leaved crops, at 50-100 g/ha. Formulation types EC. Selected products: 'Aramo' (BASF); 'Neto' (BASF)
OTHER PRODUCTS
'Equinox' (BASF); 'Honest' (BASF)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats c. 5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin and mucous membranes (rabbits); not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >5.1 mg/l.
ECOTOXICOLOGY
Birds LD50 for quail >2000 mg/kg. Fish LC50 (96 h) for rainbow trout >100 mg/l. Daphnia EC50 (48 h) >100 mg/l. Algae EC50 (72 h) for Chlorella fusca 76 mg/l. Other aquatic spp. EC10 (17 h) for Pseudomonas putida >1000 mg/l. Bees LD50 (oral and contact) >200 mg/bee. Worms LC50 (14 d) for Eisenia foetida >1000 mg/kg soil.
ENVIRONMENTAL FATE
Soil/Environment Soil DT50 (lab.) 1-9 d. Photolytic DT50 on soil surface c. 1 d.
|