|
Tembotrione
CHEMICAL NAME: Tembotrione, 2-[2-chloro-4-(methylsulfonyl)-3-[(2,2,2-(trifluoroethoxy)methyl]benzoyl]-1,3-cyclohexanedione
COMMON NAME: Tembotrione
Formula: C17H16ClF3O6S
CHEMICAL Structure:
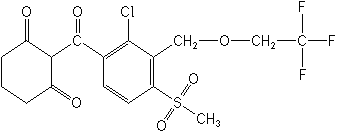
PESTICIDE TYPE: Herbicide
CHEMICAL CLASS: Triketone
US PRODUCER: Bayer CropScience LP
USE PATTERNS AND FORMULATIONS
Application Sites: Tembotrione is registered as a selective, post-emergence herbicide developed for the control of a broad spectrum of broadleaf and grassy weeds in corn.
Type of Formulations: Technical grade manufacturing use product (96.2% tembotrione) and liquid end use product (34.5% tembotrione)
Application Methods and Rates: The application rate is 0.082 lbs a.i./acre (0.092 kg a.i./ha) followed 14 days later by a second treatment at the same application rate, if needed. Applications take place between crop emergence to the V8 (more than 8 visible leaves) developmental stage of corn. Tembotrione is broadcast applied using flat-fan nozzles that provide medium to coarse spray droplets.
PHYSICAL AND CHEMICAL PROPERTIES
Description |
Value |
Melting point |
117 ºC |
pH @ 24 °C |
3.63 |
Density (g/mL @ 20 °C) |
1.56 |
Water solubility (mg/L @ 20°C) |
0.22 at pH 4
28.3 at pH 7
29.7 at pH 9 |
Solvent solubility (g/L at 20°C)
DMSO
Methylene Chloride
Acetone
Ethyl Acetate
Toluene
Hexane
Ethanol |
>600
>600
300-600
180.2
75.7
47.6
8.2 |
Vapor pressure (Torr, 20 °C) |
8.25 x 10-11 |
Dissociation constant (pKa) |
3.2 |
Octanol/water partition |
0.0430 at pH 9.0 |
coefficient |
0.0807 at pH 7.0 |
(Pow @ 23 °C ) |
144.9 at pH 2.0 |
(Pow @ 24 °C ) |
Primary: 205 |
(Pow @ 23 °C ) |
Secondary: 284 |
UV/visible absorption spectrum (nm) |
Tertiary: 240 |
IV. HUMAN HEALTH RISK ASSESSMEN
Acute Toxicity: Tembotrione has low acute toxicity via the oral, dermal and inhalation routes of exposure (Toxicity category III or IV). It is a dermal sensitizer
but not an eye or dermal irritant. The acute toxicity findings are summarized below: Summary of Acute Toxicity of Tembotrione.
Category
Acute Oral – rat LD50 > 2000 mg/kg III
Acute Dermal - rat LD50 > 2000 mg/kg III
Acute Inhalation - rat LC50 > 5.03 mg/L IV
Primary Eye Irritation –rabbit: Formulation is moderately irritating to the eye
Primary Skin Irritation –rabbit: Not an irritant to the skin IV
sensitization -guinea pig: Not a dermal sensitizer N/A
|
