|
tebuthiuron
Herbicide
HRAC C2 WSSA 7; urea
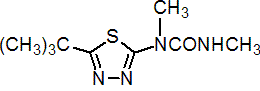
NOMENCLATURE
Common name tebuthiuron (BSI, E-ISO, (m) F-ISO, ANSI, WSSA)
IUPAC name 1-(5-tert-butyl-1,3,4-thiadiazol-2-yl)-1,3-dimethylurea
Chemical Abstracts name N-[5-(1,1-dimethylethyl)-1,3,4-thiadiazol-2-yl]-N,N'-dimethylurea
CAS RN [34014-18-1] EEC no. 251-793-7 Development codes EL-103 (Lilly)
PHYSICAL CHEMISTRY
Mol. wt. 228.3 M.f. C9H16N4OS Form Colourless, odourless solid. M.p. 162.85 °C B.p. decomp. c. 275 °C V.p. 0.04 mPa (25 ºC, gas saturation method) KOW logP = 1.82 (20 ºC) Henry 3.0 ´ 10-5 Pa m3 mol-1 (calc.) Solubility In water 2.57 g/l (20 ºC). In benzene 3.7, hexane 6.1, 2-methoxyethanol 60, acetonitrile 60, acetone 70, methanol 170, chloroform 250 (all in g/l, 25 ºC). Stability Stable at 52 ºC (highest storage temperature tested). Stable in aqueous media between pH 5 and 9. On hydrolysis, DT50 (25 ºC) >64 d (pH 3, 6 and 9).
COMMERCIALISATION
History Herbicide reported by J. F. Schwer (Proc. Br. Weed Control Conf., 12th, 1974, 2, 847). Introduced in Brazil (1974) by Eli Lilly & Co. (agrochemical interests now Dow AgroSciences). Patents GB 1266172 Manufacturers Dow AgroSciences; Milenia
APPLICATIONS
Biochemistry Photosynthetic electron transport inhibitor at the photosystem II receptor site. Mode of action Systemic soil-herbicide with low selectivity, absorbed mainly by the roots, with ready translocation. Uses Broad-spectrum herbicide for control of herbaceous and woody plants (0.6-4.5 kg/ha), annual weeds (1.3-4.5 kg/ha), many perennial grass and broad-leaved weeds (2.2-6.8 kg/ha). Uses include total control of vegetation in non-crop areas, undesirable woody plants in pastures and rangeland, and grass and broad-leaved weeds in sugar cane. Phytotoxicity Not to be applied near desirable trees or plants. Formulation types GR; WG; WP; Pellets. Selected products: 'Combine' (Dow AgroSciences); 'Spike' (Dow AgroSciences); 'Tebusan' (Dow AgroSciences)
OTHER PRODUCTS
Discontinued products: 'Bushwacker' * (Aventis); 'Graslan' * (DowElanco)
ANALYSIS
Product analysis by hplc or by glc with FID (A. Loh et al., Anal. Methods Pestic. Plant Growth Regul., 1980, 11, 351). Residues determined by glc with FPD (idem, ibid.). In drinking water, by glc with NPD (AOAC Methods, 17th Ed., 991.07). Details available from Dow AgroSciences.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male mice 528, female mice 620, male rats 477, female rats 387, rabbits 286, dogs >500, cats >200 mg/kg. Skin and eye Dermal LD50 rabbit >5000mg/kg. Not a skin or eye irritant; not a skin sensitiser. Inhalation LC50 for rats 3.696 mg/l. NOEL NOEL (2 y) for rats 40 mg/kg diet; LOEL for rats 80 mg/kg daily. Chronic systemic NOEL for mice 228 mg/kg daily. ADI 0.07 mg/kg (based on 2-generation rat reproduction study). Other No evidence of teratogenicity (rats and rabbits). Toxicity class WHO (a.i.) III; EPA (formulation) III EC classification Xn; R22| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for chickens, bobwhite quail, and mallard ducks >500 mg/kg. In 1 mo feeding trials, chickens receiving 1000 mg/kg showed no ill-effects. Fish LC50 (96 h) for rainbow trout 144, goldfish and fathead minnow >160, bluegill sunfish 112 mg/l. Daphnia LC50 297 ppm. Algae EC50 for Anabaena 4.06, Navicula 0.081, Selenastrum 0.05, Skeletonema 0.05 ppm. Other aquatic spp. EC50 for Lemna 0.135 ppm. Bees LD50 >100 mg/bee.
ENVIRONMENTAL FATE
Animals The major metabolites in mammals were formed by N-demethylation of the substituted urea side-chain (D. M. Morton & D. G. Hoffman, J. Toxicol. Environ. Health, 1976, 1, 757-768). Plants In plants, the principal metabolic pathways involve N-demethylation and hydroxylation of the tert-butyl side-chain. Soil/Environment Some microbial breakdown occurs in soil, but this is not the predominant mode of degradation. Loss due to photodecomposition and volatilisation is negligible. Half-life in soil is considerably greater in soils with low moisture content, and in high organic soils. Adsorption Freundlich K values range from 0.11 in sand (pH 7.7, o.m. 0.5%) to 1.82 in clay loam (pH 6.9, o.m. 2.0%).
|