|
sulfentrazone
Herbicide
HRAC E WSSA 14; triazolinone
NOMENCLATURE
Common name sulfentrazone (BSI, pa E-ISO, ANSI)
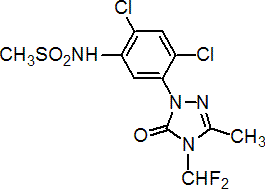
IUPAC name 2',4'-dichloro-5'-(4-difluoromethyl-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazol-1-yl)methanesulfonanilide
Chemical Abstracts name N-[2,4-dichloro-5-[4-(difluoromethyl)-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazol-1-yl]phenyl]methanesulfonamide
CAS RN [122836-35-5] Development codes F6285; FMC 97285
PHYSICAL CHEMISTRY
Mol. wt. 387.2 M.f. C11H10Cl2F2N4O3S Form Tan solid. M.p. 121-123 ºC V.p. 1.3 ´ 10-4 mPa (25 ºC) KOW logP = 1.48 S.g./density 1.21 g/ml (20 ºC) Solubility In water 0.11 (pH 6), 0.78 (pH 7), 16 (pH 7.5) (all in mg/g, 25 ºC). Soluble, to some extent, in acetone and other polar organic solvents. Stability Stable to hydrolysis. Readily photolysed in water.
COMMERCIALISATION
History Reported by W. A. van Saun et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1991, 1, 77). Introduced by FMC Corp.
APPLICATIONS
Biochemistry Protoporphyrinogen oxidase inhibitor (chlorophyll biosynthesis pathway). Mode of action Herbicide absorbed by the roots and foliage, with translocation primarily in the apoplasm, and limited movement in the phloem. Uses Control of annual broad-leaved weeds, some grasses and Cyperus spp. in soya beans, sugar cane and tobacco. Applied pre-emergence or pre-plant incorporated. Formulation types SC; WG. Selected products: 'Authority' (USA) (FMC); 'Boral' (Brazil) (FMC); 'Capaz' (Latin America) (FMC)
OTHER PRODUCTS
'Cover' (DuPont) mixtures: 'Authority BL' (+ metribuzin) (FMC); 'Authority Broadleaf' (+ chlorimuron-ethyl) (FMC); 'Authority One-Pass' (+ clomazone) (FMC); 'Command Xtra' (+ clomazone) (FMC); 'Gauntlet' (+ cloransulam-methyl) (FMC); 'Canopy XL' (+ chlorimuron-ethyl) (DuPont) Discontinued products: 'Spartan' * (FMC)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 2855 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Non-irritating to skin; mild eye irritant (rabbits). Non-sensitising to skin (guinea pigs). Inhalation LC50 (4 h) for rats >4.14 mg/l. NOEL 10 mg/kg daily (rat teratology study). Other Non-mutagenic in the Ames test, mouse lymphoma and in vivo mouse micronucleus assay. Toxicity class EPA (formulation) III
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >2250 mg/kg. Dietary LC50 (8 d) for ducks and quail >5620 mg/kg. Fish LC50 (96 h) for bluegill sunfish 93.8, rainbow trout >130 mg/l. Daphnia LC50 (48 h) 60.4 mg/l.
ENVIRONMENTAL FATE
Animals In rats, nearly all of administered sulfentrazone is excreted in the urine within 72 h. Plants In soya beans, over 95% of the parent sulfentrazone is metabolised to the non-polar, ring-hydroxymethyl analogue within 12 hours. This analogue is also rapidly converted, over the same time period, to three polar metabolites, two of which are glycosidic derivatives and one a non-glycoside metabolite. Soil/Environment Stable in soil (DT50 18 mo). In water, stable to hydrolysis (pH 5-9), but readily undergoes photolysis (DT50 <0.5 d). Low affinity for organic matter (Koc 43), but is mobile only in soils with high sand content. Low potential to bioaccumulate.
|