siduron
Herbicide
HR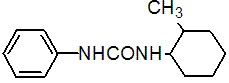 AC C2 WSSA 7; urea AC C2 WSSA 7; urea
NOMENCLATURE
Common name siduron (BSI, E-ISO, (m) F-ISO, ANSI, WSSA, JMAF)
IUPAC name 1-(2-methylcyclohexyl)-3-phenylurea
Chemical Abstracts name N-(2-methylcyclohexyl)-N'-phenylurea
CAS RN [1982-49-6] Development codes DuPont 1318
PHYSICAL CHEMISTRY
Composition Tech. is >98%. Mol. wt. 232.3 M.f. C14H20N2O Form Colourless crystals. M.p. 133-138 ºC V.p. 5.3 ´ 10-4 mPa (25 °C) KOW logP = 3.8 Henry 6.8 ´ 10-6 Pa m3 mol-1 (calc.) S.g./density 1.08 (25 ºC) Solubility In water 18 mg/l (25 ºC). In dimethylacetamide 367, dimethylformamide 260, ethanol 160, isophorone, dichloromethane 118 (all in g/kg, 25 ºC). Stability Stable up to its melting point and in neutral aqueous solutions. Decomposes slowly in acid and alkaline media. DT50 >>30 d (pH 5, 7, 9, 25 °C).
COMMERCIALISATION
History Herbicide reported by R. W. Varner et al. (Proc. Br. Weed Control Conf., 7th, 1964, p. 38). Introduced by E. I. du Pont de Nemours and Co. (who no longer market it). Marketed by Gowan since 1994. Patents US 3309192 Manufacturers Raschig
APPLICATIONS
Biochemistry Photosynthetic electron transport inhibitor at the photosystem II receptor site. Mode of action Selective herbicide, absorbed by the roots, with translocation in the xylem. Uses Pre-emergence control of Digitaria spp. and annual grass weeds in turf farms, grass seed production, and established turf. Applied at 2.24-10 kg/ha in newly seeded grass and 9-13.45 kg/ha in established turf. Phytotoxicity Bermuda grass and certain bent grasses may be injured when applied pre-emergence. Formulation types WP. Selected products: 'Tupersan' (Gowan)
ANALYSIS
Product analysis by hplc. Residues determined by colorimetry (R. L. Dalton & H. L. Pease, J. Assoc. Off. Agric. Chem., 1962, 45, 377).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >7500 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >5500 mg/kg (maximum feasible dose). Inhalation LC50 (4 h) for rats >5.8 mg/l. NOEL (2 y) for rats 500, dogs 2500 mg/kg diet. ADI Not necessary; non-food uses only. Toxicity class WHO (a.i.) U; EPA (formulation) III (WP)
ECOTOXICOLOGY
Birds Dietary LC50 (8 d) for mallard ducklings and bobwhite quail >10 000 mg/kg diet. Fish LC50 (96 h) for rainbow trout 14, bluegill sunfish 16 mg/l. Daphnia EC50 (48 h) 18 mg/l. Algae EC50 (120 h) for Selenastrum capricornutum 250 mg/l.
ENVIRONMENTAL FATE
Plants In studies with 14C-labelled siduron, no metabolites were detected in barley plants after an 8-day absorption period. Soil/Environment In soil, resists leaching. Microbial degradation occurs, DT50 c. 120-150 d; metabolites include methylcyclohexylurea, methylcyclohexylamine, phenylurea and aniline. In water, photolysis DT50 290 d (natural sunlight).
|