|
S-metolachlor
Herbicide
chloroacetamide
NOMENCLATURE
Common name S-metolachlor (BSI, pa ISO)
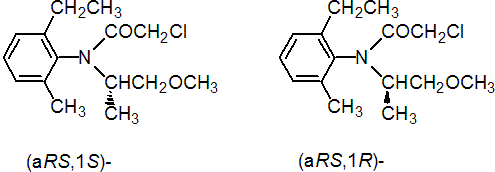
IUPAC name A mixture of (aRS,1S)-2-chloro-6'-ethyl-N-(2-methoxy-1-methylethyl)aceto-o-toluidide and (aRS,1R)-2-chloro-6'-ethyl-N-(2-methoxy-1-methylethyl)aceto-o-toluidide in the proportion 80-100% to 20-0%
Chemical Abstracts name A mixture of (S)-2-chloro-N-(2-ethyl-6-methylphenyl)-N-(2-methoxy-1-methylethyl)acetamide and (R)-2-chloro-N-(2-ethyl-6-methylphenyl)-N-(2-methoxy-1-methylethyl)acetamide in the proportion 80-100% to 20-0%
CAS RN [87392-12-9] (S)- isomer; [178961-20-1] (R)- isomer Development codes CGA 77102 [(aRS,1S)- isomers] (Novartis); CGA 77101 [(aRS,1R)- isomers] (Novartis)
PHYSICAL CHEMISTRY
Composition A mixture of isomers in the proportion 80-100% (S)- to 20-0% (R)- Mol. wt. 283.8 M.f. C15H22ClNO2 Form Clear yellow to brownish liquid, with an unspecific odour. M.p. -61.1 °C B.p. c. 334 °C/760 mmHg V.p. 3.7 mPa (25 °C) KOW logP = 3.05 (20 °C) S.g./density 1.117 (20 °C) Solubility In water 480 mg/l (25 °C) Stability Hydrolysis DT50 >200 d (pH 7-9, 20 °C). F.p. 190 °C (EEC A9)
COMMERCIALISATION
History Reported by T. R. Dill et al. (Proc. North Centr. Weed Sci. Soc., 1996, 51, 58-59). Introduced by Novartis Crop Protection AG (now Syngenta AG). Patents US 5002606; EP 77755 Manufacturers Syngenta
APPLICATIONS
Biochemistry Cell division inhibitor; more recent research suggests chloroacetamides may inhibit synthesis of very long chain fatty acids (J. Schmalfuss et al., Abstr. Meeting WSSA, Toronto, 40, 117-118, 2000; P. Böger, Abstr. III Int. Weed Control Congr., Brazil 2000). Maize tolerance of chloroacetamides is attributed to rapid detoxification by glutathione S-transferases. Mode of action Selective herbicide, absorbed predominantly by the hypocotyls and shoots; inhibits germination Uses Control of annual grasses (Echinochloa, Digitaria, Setaria, Brachiaria, Panicum, and Cyperus) and some broad-leaved weeds (Amaranthus, Capsella, Portulaca) in maize, sorghum, cotton, sugar beet, fodder beet, sugar cane, potatoes, soya beans, peanuts, sunflowers, various vegetables, and pulse crops. Applied pre-plant incorporated, pre-emergence or early post-emergence, at 0.8-1.6 kg/ha. Often used in combination with broad-leaved herbicides, to extend spectrum of activity. Phytotoxicity Tolerated by most broad-leaved crops, maize, sorghum (when safened wth fluxofenim or oxabetrinil). Formulation types EC; FW; GR; SC. Selected products: 'Dual Gold' (Syngenta); 'Dual Magnum' (USA) (Syngenta); mixtures: 'Bicep II Magnum' (+ atrazine+ benoxacor) (USA) (Syngenta); 'Bicep Magnum' (+ atrazine) (USA) (Syngenta); 'Camix' (+ benoxacor+ mesotrione) (Syngenta); 'Dual II Magnum' (+ benoxacor) (USA) (Syngenta); 'Lumax' (+ benoxacor+ mesotrione) (Syngenta)
OTHER PRODUCTS
'Medal' (Syngenta); 'Cinch' (USA) (DuPont) mixtures: 'Bicep Magnum TR' (+ atrazine+ flumetsulam) (Syngenta); 'Boundary' (+ metribuzin) (Syngenta); 'Gardo Gold' (+ terbuthylazine) (Syngenta); 'Gardoprim Plus Gold' (+ terbuthylazine) (Syngenta); 'Cinch ATZ' (+ atrazine) (USA) (DuPont); 'Twin Pack Gold' (+ flurochloridone) (Agan) Discontinued products mixtures: 'Primextra II Magnum' * (+ atrazine+ benoxacor) (Syngenta); 'Primextra' * (+ atrazine) (Syngenta)
ANALYSIS
By glc or hplc; details available from Syngenta.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 2672 mg/kg. Skin and eye Acute dermal LD50 for rabbits >2000 mg/kg; non-irritant to skin and eyes (rabbits); may cause sensitisation by skin contact (guinea pigs). Inhalation LC50 (4 h) for rats >2910 mg/m3. Toxicity class WHO (a.i.) III (company classification)
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail and mallard ducks >2510 mg/kg. Dietary LC50 (8 d) for bobwhite quail and mallard ducks >5620 ppm. Fish LC50 (96 h) for rainbow trout 1.2, bluegill sunfish 3.2 mg/l. Daphnia LC50 (48 h) 11.2 mg/l. Algae EC50 (72 h) 0.006-0.14 mg/l. Bees LD50 (oral) >0.085 mg/bee; (contact) >0.2 mg/bee. Worms LC50 (14 d) for earthworms 570 mg/kg soil.
ENVIRONMENTAL FATE
Animals Rapidly oxidised by rat liver oxygenases via dechlorination, O-demethylation and side-chain oxidation; conjugation by glutathione S-transferases. Plants Metabolism involves dechlorination and conjugation to glutathione S-transferases, followed by further degradation to polar, water-soluble, non-volatile metabolites. Soil/Environment Major aerobic metabolites are derivatives of oxanilic and sulfonic acids; DT50 (field) 11-30 d; DT90 (field) 36-90 d.
|