|
quizalofop-P-ethyl
Herbicide
HRAC A WSSA 1; aryloxyphenoxypropionate
NOMENCLATURE
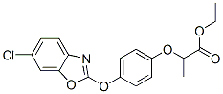
quizalofop-P-ethyl
Common name quizalofop-P-ethyl (BSI, draft E-ISO)
IUPAC name ethyl (R)-2-[4-(6-chloroquinoxalin-2-yloxy)phenoxy]propionate
CAS RN [100646-51-3] Development codes D(+) NC-302 (Nissan); DPX-79376 (DuPont)
PHYSICAL CHEMISTRY
quizalofop-P-ethyl
Mol. wt. 372.8 M.f. C19H17ClN2O4 Form White, crystalline, odourless solid. M.p. 76.1-77.1 ºC B.p. 220 ºC/26.6 Pa V.p. 1.1 ´ 10-4 mPa (20 ºC) KOW logP = 4.61 (23? ºC) Henry 6.7 ´ 10-5 Pa m3 mol-1 (calc.) S.g./density 1.36 g/cm3 Solubility In water 0.61 mg/l (20 ºC). In acetone, ethyl acetate and xylene >250, 1,2-dichloroethane >1000 (all in g/l, 22-23 °C); in methanol 34.87, n-heptane 7.168 (both in g/l, 20 °C). Stability Stable in neutral and acidic media, but unstable in alkaline media; DT50 <1 d (pH 9). Stable at high temperatures and in organic solvents. Specific rotation [a]D20 +35.9?(20 ºC)
COMMERCIALISATION
History The herbicidal enantiomer ester quizalofop-P-ethyl introduced by Nissan Chemical Industries, Ltd and quizalofop-P-tefuryl ((?-tetrahydrofurfuryl ester) (A. R. Bell & A. S. Peddie, Proc. 1989 Br. Crop Prot. Conf. - Weeds, 1, 65) by Uniroyal Chemical Co., Inc. (now part of Crompton Corp.).
quizalofop-P-ethyl
Manufacturers Fengle; IPESA; Nissan; Sharda
APPLICATIONS
Biochemistry Fatty acid synthesis inhibitor (inhibition of acetyl CoA carboxylase) Mode of action Systemic herbicide, absorbed from the leaf surface, with translocation throughout the plant, moving in both the xylem and phloem, and accumulating in the meristematic tissue.
quizalofop-P-ethyl
Uses Selective post-emergence control of annual and perennial grass weeds in potatoes, soya beans, sugar beet, peanuts, oilseed rape, sunflowers, vegetables, cotton, and flax. Phytotoxicity Most non-graminaceous crops are tolerant. Formulation types EC; SC. Compatibility Can be used in combination with post-emergence broad-leaved herbicides. Selected products: 'CoPilot' (Nissan); 'Pilot D' (Nissan); 'Pilot Super' (Nissan); 'Targa D+' (Nissan); 'Targa Super' (Nissan); 'Assure II' (USA) (DuPont); 'Herban LPU' (IPESA); 'Leopard' (Makhteshim-Agan); 'Mostar' (IPESA)
OTHER PRODUCTS
quizalofop-P-ethyl
'Matador' (FMC); 'Nervure Super' (KenoGard); 'Omega' (Argentina) (DuPont); 'Sceptre' (Bayer CropScience); 'Sheriff' (DuPont); 'Targa Prestige' (Bayer CropScience)
ANALYSIS
quizalofop-P-ethyl
Product by hplc. Residues by glc. Details from Nissan.
MAMMALIAN TOXICOLOGY
quizalofop-P-ethyl
Oral Acute oral LD50 for male rats 1210, female rats 1182, male mice 1753, female mice 1805 mg/kg. NOEL (90 d) for rats 7.7 mg/kg daily.
ECOTOXICOLOGY
quizalofop-P-ethyl
Birds Acute oral LD50 for mallard ducks and bobwhite quail >2000 mg/kg. Fish LC50 (96 h) for rainbow trout >0.5 mg/l. Daphnia LC50 (48 h) 0.29 mg/l. Algae Assumed similar to racemate. Bees Assumed similar to racemate. Worms LC50 >1000 mg/kg.
ENVIRONMENTAL FATE
quizalofop-P-ethyl
Animals The degradation pattern is the same as that for quizalofop-ethyl. Plants The degradation pattern is the same as that for quizalofop-ethyl. Soil/Environment In soil, degrades rapidly to quizalofop-P; DT50 £1 d.
|
