|
quinoxyfen
Fungicide
FRAC 13, E1; quinoline
NOMENCLATURE
Common name quinoxyfen (BSI, pa ISO, ANSI)
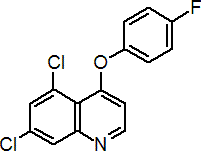
IUPAC name 5,7-dichloro-4-quinolyl 4-fluorophenyl ether
Chemical Abstracts name 5,7-dichloro-4-(4-fluorophenoxy)quinoline
CAS RN [124495-18-7] Development codes DE-795; XDE-795 (both Dow); LY211795
PHYSICAL CHEMISTRY
Composition Tech. is ³97%. Mol. wt. 308.1 M.f. C15H8Cl2FNO Form Off-white solid. M.p. 106-107.5 °C V.p. 1.2 ´ 10-2 mPa (20 ºC); 2.0 ´ 10-2 mPa (25 ºC). KOW logP = 4.66 (pH c. 6.6, 20 °C) Henry 3.19 ´ 10-2 Pa m3 mol-1 (calc.) S.g./density 1.56 Solubility In water 116 mg/l (pH 6.45, 20 ºC). In acetone 116, dichloromethane 589, ethyl acetate 179, methanol 21.5, n-octanol 37.9, toluene 272, hexane 9.64, xylene 200 (all in g/l, 20 °C). Stability In dark at 25 °C, stable to hydrolysis at pH 7 and 9; DT50 75 d (pH 4). Degraded more rapidly in light. F.p. >100 °C
COMMERCIALISATION
History Reported by C. Longhurst et al. (Proc. Br. Crop Prot. Conf. - Pests Dis., 1996, 1, 27). Introduced by DowElanco (now Dow AgroSciences). First provisional registrations were obtained in Europe in 1996. Manufacturers Dow AgroSciences
APPLICATIONS
Biochemistry Growth signal disruptor. Shown not to be a sterol demethylation nor a mitochondrial electron transport inhibitor, nor to inhibit dihydro-orotate dehydrogenase (an enzyme involved in the biosynthesis of pyrimidines). Mode of action Mobile, protectant fungicide acting through inhibition of appressorial development; not effective as an eradicant. Active through systemic acropetal and basipetal movement, and by vapour transfer. Uses For the control of cereal powdery mildew (Erysiphe graminis), at 100-150 g/ha; offers long-term (up to 70 d) protection. No cross-resistance with current mildewicides such as azoles, morpholines or strobilurins. Launched in grapes for the control of powdery mildew (Uncinula necator), at 50-75 g/ha. Under development in selected vegetable crops, hops and sugar beet for the control of powdery mildews. Phytotoxicity Injury has been reported in cucurbits grown under cover. Formulation types SC. Selected products: 'Fortress' (Dow AgroSciences); 'Quintec' (Dow AgroSciences)
OTHER PRODUCTS
'Abir' (Dow AgroSciences); 'Apres' (Dow AgroSciences); 'Arius' (Dow AgroSciences); 'Atlas' (Dow AgroSciences); 'Crystal' (Dow AgroSciences); 'Elios' (Dow AgroSciences); 'Erysto' (Dow AgroSciences); 'Helios' (Dow AgroSciences); 'Legend' (Dow AgroSciences); 'After' (Me2) mixtures: 'Aldus' (+ cyproconazole) (Dow AgroSciences); 'Divora' (+ cyproconazole) (Dow AgroSciences, Syngenta); 'Fortress Duo' (+ fenpropimorph) (Dow AgroSciences); 'Fortress Top' (+ fenpropimorph) (Dow AgroSciences); 'Orka' (+ fenpropimorph) (Dow AgroSciences); 'Porter' (+ fenarimol) (Dow AgroSciences); 'Sonic' (+ fenarimol) (Dow AgroSciences); 'Trisave' (+ fenarimol) (Dow AgroSciences); 'Vento' (+ fenarimol) (Dow AgroSciences)
ANALYSIS
Residue analysis in plant, animal and soil matrices by gc with mass selective detection. Analysis in drinking water by hplc with uv detection. Details available from Dow AgroSciences.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Mild eye irritant, not a skin irritant (rabbits). Skin sensitisation (guinea pigs) depends on the test. Inhalation LC50 for rats >3.38 mg/l. NOEL Based on 52 week dog study, 2 year rat carcinogenicity study and rat reproduction study, 20 mg/kg b.w. daily ADI 0.2 mg/kg. Other Not mutagenic, teratogenic or oncogenic. Toxicity class WHO (a.i.) U EC classification R43| N; R50, R53
ECOTOXICOLOGY
Birds LD50 for bobwhite quail >2250 mg/kg. LC50 (5 d) for bobwhite quail and mallard ducks >5620 mg/kg diet. Fish LC50 (96 h) for rainbow trout 0.27, bluegill sunfish >0.28, common carp 0.41 mg/l. Daphnia EC50 (48 h) 0.08 mg/l. Algae EbC50 (72 h) 0.058 mg/l. Other aquatic spp. NOEC (28 d) for Chironomus riparius 0.128 mg/l (water spiked). Bees LD50 (oral and contact) >100 mg/bee. Worms LC50 (14 d) >923 mg/kg soil. Other beneficial spp. Harmless to most non-target arthropod species in laboratory tests (IOBC). Little or no effect in field studies; no effect on soil micro-organisms at 4000 g/ha. See M. Miles & C. Longhurst Proc. Br. Crop Prot. Conf. - Pests Dis., 2000, 1, 371.
ENVIRONMENTAL FATE
Slowly biodegraded in plants, animals and soil. However, hydrolysis under acidic conditions, aqueous photolysis, and strong sorption to soils/sediment are important degradation/dissipation processes. Quinoxyfen and its residues have negligible leaching potential and will not pose a threat to groundwater. Animals Metabolism in goat and hen studied (G. L. Reeves et al., Proc. Br. Crop Prot. Conf. - Pests Dis., 1996, 3, 1169). Plants Only slightly metabolised in wheat, with low residues found in the crop (idem, ibid.). Extensively photodegraded on the wheat leaf surface, giving multiple polar degradation products. On grapes and cucumbers grown under glass, the main residue was unchanged quinoxyfen. Soil/Environment Soil DT50 (field) 11-454 d (biphasic, 10 trials); DT90 (field) >1 y, but does not accumulate under normal practice. DT50 (lab, aerobic) 106-508 d (7 soils, 20-25°C); DT50 (lab, anaerobic) 289 d (20°C). Minimal soil photolysis (est. DT50 (field) >1 y). The main metabolite (3-hydroxyquinoxyfen) is formed by hydroxylation of the quinoline ring, and the minor metabolite (5,7-dichloro-4-hydroxyquinoline, DCHQ) is formed by cleavage of ether bridge, especially in acidic soil. Koc 15 415-75 900; quinoxyfen and its residues have no significant leaching potential. Water For aqueous hydrolysis in the dark, see Stability. Photolysis in water is more significant, DT50 1.7 h (June), 22.8 h (December). In dark water/sediment systems, quinoxyfen rapidly migrates from water to sediment and is moderately degraded, DT50 (lab) 35-150 d, to give 3-hydroxyquinoxyfen. Air No significant volatile losses are expected following application. Any small amounts present in air are estimated to degrade with atmospheric t½ 1.88 d.
|