|
pyriproxyfen
Insecticide
IRAC 7C; juvenile hormone mimic
NOMENCLATURE
Common name pyriproxyfen (BSI, draft E-ISO); no name (Sweden)
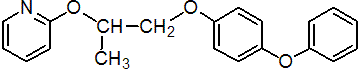
IUPAC name 4-phenoxyphenyl (RS)-2-(2-pyridyloxy)propyl ether
Chemical Abstracts name 2-[1-methyl-2-(4-phenoxyphenoxy)ethoxy]pyridine
CAS RN [95737-68-1] unstated stereochemistry Development codes S-9318; S-31183 (both Sumitomo)
PHYSICAL CHEMISTRY
Mol. wt. 321.4 M.f. C20H19NO3 Form Colourless crystals; (tech. is a pale yellow, waxy solid, with a faint odour). M.p. 47 ºC V.p. <0.013 mPa (23 °C) KOW logP = 5.37 S.g./density 1.24 (25 ºC) Solubility In hexane 400, methanol 200, xylene 500 (all in g/kg, 20-25 ºC). F.p. 119 °C (Pensky-Martens closed test)
COMMERCIALISATION
History Insecticide introduced by Sumitomo Chemical Co., Ltd. First registered in Japan in 1989 for public health uses; first registered for agricultural use in Japan in 1995. Manufacturers Sumitomo
APPLICATIONS
Mode of action Insect growth regulator; suppressor of embryogenesis, inhibitor of metamorphosis and inhibitor of reproduction. Uses Control of public health insect pests (flies, beetles, midges, mosquitoes); applied to breeding sites (swamps, livestock houses, etc.). Also used for control of whitefly and thrips. Formulation types EC; GR; WG. Selected products: 'Sumilarv' (public health) (Sumitomo)
OTHER PRODUCTS
'Admiral' (Sumitomo); 'Atominal' (Sumitomo, Philagro); 'Epingle' (Sumitomo); 'Juvinal' (Sumitomo, Philagro); 'Lano' (Sumitomo); 'Nemesis' (Sumitomo); 'Distance' (Valent); 'Esteem' (Valent); 'Knack' (Valent, Sumitomo); 'Nylar' (cockroach and flea control) (MGK); 'Seize' (Valent)
ANALYSIS
Details from Sumitomo.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 86, 88, 92 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Not an irritant to skin and eyes (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >1300 mg/m3. ADI (JMPR) 0.1 mg/kg b.w. [1999, 2001]. Toxicity class WHO (a.i.) U
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks and bobwhite quail >2000 mg/kg. Dietary LC50 for bobwhite quail and mallard ducks >5200 ppm. Fish LC50 (96 h) for rainbow trout >0.325 mg/l. Daphnia EC50 (48 h) 0.40 mg/l. Algae EC50 (72 h) for Selenastrum capricornutum 0.064 mg/l.
|