|
pyriminobac-methyl
Herbicide
HRAC B WSSA 2; pyrimidinyloxybenzoic
NOMENCLATURE
Common name pyriminobac-methyl (BSI, pa ISO)
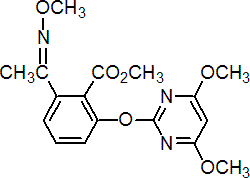
IUPAC name methyl 2-(4,6-dimethoxy-2-pyrimidinyloxy)-6-(1-methoxyiminoethyl)benzoate
Chemical Abstracts name methyl 2-[(4,6-dimethoxypyrimidin-2-yl)oxy]-6-[1-(methoxyimino)ethyl]benzoate
CAS RN [136191-64-5] methyl ester; [136191-56-5] acid Development codes KIH-6127; KUH-920 (both Kumiai)
PHYSICAL CHEMISTRY
Composition Tech. is >93%: (E)- isomer 75-78%, (Z)- isomer 20-11%. Mol. wt. 361.4 M.f. C17H19N3O6 Form White powder; (tech., pale yellow grains). M.p. Tech. 105 ºC; pure (E)- isomer 107-109 °C; pure (Z)- isomer 70 °C V.p. (E)- isomer 3.5 ´ 10-2 mPa (25 °C); (Z)- isomer 2.681 ´ 10-2 mPa (25 °C) KOW (E)- isomer, logP = 2.98 (21.5 °C); (Z)- isomer, logP = 2.70 (20.6 °C) S.g./density (E)- isomer 1.3868; (Z)- isomer 1.2734 (both 20 °C) Solubility (E)- isomer: in water 0.00925, methanol 14.6 (both in g/l, 20 ºC). (Z)- isomer: in water 0.175, methanol 14.0 (both in g/l, 20 ºC). Stability Stable in water (>1 y, pH 4-9), to light, and to heat (not decomposed after 14 d at 55 °C)
COMMERCIALISATION
History Reported by Kumiai Chemical Industry Co., Ltd (R. Hanai et al., Proc. Br. Crop Prot. Conf. - Weeds, 1993, 1, 47). Introduced by Kumiai in Japan in 1996. Patents US 5118339 Manufacturers Ihara/Kumiai
APPLICATIONS
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Mode of action Selective, systemic herbicide, absorbed by foliage. Uses Selective control of barnyardgrass (Echinochloa spp.) in paddy rice; applied early post-emergence, at 30-60 g/ha. Formulation types GR; WP. Selected products: mixtures: 'Patful' (+ bensulfuron-methyl+ oxaziclomefone) (Kumiai); 'Prosper' (+ bensulfuron-methyl+ mefenacet) (Kumiai); 'Topgun' (+ bensulfuron-methyl+ bromobutide+ pentoxazone) (Kumiai)
OTHER PRODUCTS
Discontinued products mixtures: 'Tabijin A' * (+ azimsulfuron+ bensulfuron-methyl+ cyhalofop-butyl+ pretilachlor) (Kumiai)
ANALYSIS
Product and residue analysis by glc. Details fromKumiai.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Slightly irritant to skin and eyes (rabbits). Inhalation LC50 (4 h, 14 d observation) for rats >5.5 mg/l air. NOEL (2 y) for male rats 0.9, female rats 1.2, male mice 8.1, female mice 9.3 mg/kg b.w. daily. ADI 0.009 mg/kg. Other Non-mutagenic in the Ames Test. Non-teratogenic (rats, rabbits). Toxicity class WHO (a.i.) U
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Dietary LC50 (5 d) for bobwhite quail and mallard ducks >5200 mg/kg diet. Fish LC50 (96 h) for carp 30.9, rainbow trout 21.2 mg/l. Daphnia LC50 (24 h) for D. carinata >20 mg/l. Bees LD50 (24 h, oral and contact) >200 mg/bee. Worms NOEL (14 d) for Eisenia foetida >1000 mg/kg soil.
ENVIRONMENTAL FATE
Animals Almost all of dosed 14C was excreted in urine and faeces. Many metabolites were detected. Plants c. 10% of soil-applied 14C-pyriminobac-methyl (4-leaf stage, rice) was distributed throughout the plant; at harvest, residues were mostly in the straw. Soil/Environment Koc ((E)- isomer) 972, ((Z)- isomer) 499.
|