|
pyriftalid
Herbicide
HRAC B WSSA 2; pyrimidinyloxybenzoic
NOMENCLATURE
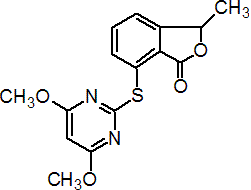
Common name pyriftalid (BSI, pa ISO)
IUPAC name (RS)-7-(4,6-dimethoxypyrimidin-2-ylthio)-3-methyl-2-benzofuran-1(3H)-one
Chemical Abstracts name 7-[(4,6-dimethoxy-2-pyrimidinyl)thio]-3-methyl-1(3H)-isobenzofuranone
CAS RN [135186-78-6] Development codes CGA 279233 (Ciba-Geigy)
PHYSICAL CHEMISTRY
Mol. wt. 318.4 M.f. C15H14N2O4S Form White, odourless solid. M.p. 163.4 °C B.p. decomp. 300 °C V.p. 2.2 ´ 10-5 mPa (25 °C) KOW logP = 2.6 S.g./density 1.44 Solubility In water 1.8 mg/l (25 °C)
COMMERCIALISATION
History Discovered by Dr Maag AG (now Syngenta AG). Reported by C. Lüthy, "New Chemistries for Crop Protection", SCI Symposium, London; 19th June, 2000. Under development by Syngenta AG. Patents WO 91/05781 Manufacturers Syngenta
APPLICATIONS
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Activity appears to derive by conversion to the ring-open salicylic acid form. Mode of action Mainly taken up through the roots and the stem base of susceptible weeds following application under flooded conditions. Uses Under development for grass weed control in rice, at 100-300 g/ha. Selected products: mixtures: 'Apiro Ace' (+ cinosulfuron) (Syngenta)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Inhalation LC50 for rats >5540 mg/m3. Toxicity class WHO (a.i.) III
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 1505 mg/kg. Fish LC50 for rainbow trout 81 ppm. Daphnia LC50 (48 h) 0.83 mg/l. Algae Scenedesmus subspicatus 82.12 mg/l. Bees LD50 (contact) >100 mg/bee.
ENVIRONMENTAL FATE
Plants No detectable residues. Soil/Environment Rapidly degraded; DT50 5-20 days.
|