|
pyrazosulfuron-ethyl
Herbicide
HRAC B WSSA 2; sulfonylurea
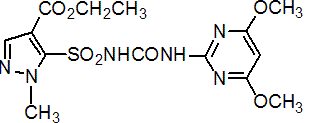
NOMENCLATURE
pyrazosulfuron-ethyl
Common name pyrazosulfuron-ethyl
IUPAC name ethyl 5-(4,6-dimethoxypyrimidin-2-ylcarbamoylsulfamoyl)-1-methylpyrazole-4-carboxylate
Chemical Abstracts name ethyl 5-[[[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]amino]sulfonyl]-1-methyl-1H-pyrazole-4-carboxylate
CAS RN [93697-74-6] Development codes NC-311 (Nissan)
pyrazosulfuron
Common name pyrazosulfuron (BSI, draft E-ISO, (m) draft F-ISO)
IUPAC name 5-(4,6-dimethoxypyrimidin-2-ylcarbamoylsulfamoyl)-1-methylpyrazole-4-carboxylic acid
Chemical Abstracts name 5-[[[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]amino]sulfonyl]-1-methyl-1H-pyrazole-4-carboxylic acid
CAS RN [98389-04-9]
PHYSICAL CHEMISTRY
pyrazosulfuron-ethyl
Mol. wt. 414.4 M.f. C14H18N6O7S Form Colourless crystals. M.p. 177.8-179.5 °C V.p. 4.2 ´ 10-5 mPa (25 ºC) KOW logP = 3.16 (hplc method) S.g./density 1.46 (20 ºC) Solubility In water 9.76 mg/l (20 ºC). In methanol 4.32, hexane 0.0185, benzene 15.6, chloroform 200, acetone 33.7 (all in g/l, 20 ºC). Stability Stable at 50 ºC for 6 months. Relatively stable at pH 7. Unstable in acidic or alkaline media. pKa 3.7
pyrazosulfuron
Mol. wt. 386.3 M.f. C12H14N6O7S
COMMERCIALISATION
History Pyrazosulfuron-ethyl reported as a herbicide by S. Kobayashi (Jpn. Pestic. Inf., 1989, No. 55, p. 17). Introduced by Nissan Chemical Industries, Ltd in 1990. Manufacturers JIE; Nissan; Sannong; Shenyang
APPLICATIONS
pyrazosulfuron-ethyl
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential amino acids valine and isoleucine, hence stopping cell division and plant growth. Selectivity derives from rapid metabolism (demethylation of methoxy group) in the crop. Metabolic basis of selectivity in sulfonylureas reviewed (M. K. Koeppe & H. M. Brown, Agro-Food-Industry, 6, 9-14 (1995)). Mode of action Systemic herbicide, absorbed by roots and/or leaves and translocated to the meristems. Uses Control of annual and perennial broad-leaved weeds and sedges, pre- or post-emergence in wet-sown and transplanted rice crops, at 15-30 g/ha. Formulation types GR; SC; WG; WP. Selected products: 'Agreen' (Nissan); 'Sirius' (Nissan); 'Billy' (Sanonda); mixtures: 'Sparkstar G' (+ dimethametryn+ esprocarb+ pretilachlor) (Nissan, Syngenta); 'Act' (+ mefenacet) (Nihon Bayer, Nissan, Yashima)
OTHER PRODUCTS
pyrazosulfuron-ethyl
'Acord' (Crystal) mixtures: 'Die-Hard' (+ cafenstrole) (Nissan, Yashima); 'Revolver' (+ cyhalofop-butyl+ mefenacet) (Nissan); 'Striker' (+ cafenstrole+ cyhalofop-butyl) (Nissan, Yashima); 'Technostar' (+ cafenstrole) (Nissan); 'Agrostar' (+ butamifos+ cyhalofop-butyl) (Sumitomo); 'Contract' (+ esprocarb) (Syngenta); 'Doublestar' (+ fentrazamide) (Bayer CropScience); 'Grassy' (+ indanofan) (Nihon Nohyaku); 'Kirifuda' (+ indanofan) (Nihon Nohyaku); 'Nondoctor' (+ indanofan) (Korea) (Nihon Nohyaku); 'Pyrasulfonil' (+ propanil) (Crystal); 'Regnet' (+ indanofan) (Nihon Nohyaku); 'Staabo' (+ pentoxazone) (Kaken, Nissan); 'Sunwell' (+ etobenzanid) (Hodogaya, Nissan) Discontinued products mixtures: 'Trebiace' * (+ indanofan) (turf use) (Mitsubishi Chemical)
ANALYSIS
Product by hplc. Methods for sulfonylurea residues in crops, soil and water reviewed (A. C. Barefoot et al., Proc. Br. Crop Prot. Conf. - Weeds, 1995, 2, 707). Details from Nissan.
MAMMALIAN TOXICOLOGY
pyrazosulfuron-ethyl
Oral Acute oral LD50 for rats and mice >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin and eyes (rabbits). Non-sensitising to skin (guinea pigs). Inhalation LC50 for rats >3.9 mg/l air. NOEL (78 w) for mice 4.3 mg/kg daily. ADI 0.043 mg/kg. Other Non-mutagenic in the Ames test. Non-teratogenic in rats and rabbits. Toxicity class WHO (a.i.) U
ECOTOXICOLOGY
pyrazosulfuron-ethyl
Birds Acute oral LD50 for bobwhite quail >2250 mg/kg. Fish LC50 (96 h) for rainbow trout and bluegill sunfish >180 mg/l; (48 h) for carp >30 mg/l. Daphnia TLm (3 h) >40 ppm. Bees LD50 (contact) >100 mg/bee.
ENVIRONMENTAL FATE
Animals In rats, after 48 h, 80% of applied pyrazosulfuron-ethyl is excreted in urine and faeces. The major metabolic reaction is demethylation of the methoxy group. Soil/Environment In soil, DT50 <15 d. In water, DT50 in buffer solution (pH 7), paddy fields or river water are c. 28 d.
|