|
pyrazophos
Fungicide
FRAC 6, F2; phosphorothiolate
NOMENCLATURE
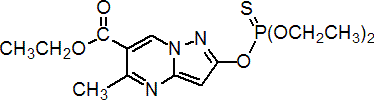
Common name pyrazophos (BSI, E-ISO, (m) F-ISO)
IUPAC name ethyl 2-diethoxyphosphinothioyloxy-5-methylpyrazolo[1,5-a]pyrimidine-6-carboxylate; O,O-diethyl O-6-ethoxycarbonyl-5-methylpyrazolo[1,5-a]pyrimidin-2-yl phosphorothioate
Chemical Abstracts name ethyl 2-[(diethoxyphosphinothioyl)oxy]-5-methylpyrazolo[1,5-a]pyrimidine-6-carboxylate
CAS RN [13457-18-6] EEC no. 236-656-1 Development codes Hoe 02 873 (Hoechst)
PHYSICAL CHEMISTRY
Composition Tech. grade is ³94% pure. Mol. wt. 373.4 M.f. C14H20N3O5PS Form Colourless crystals. M.p. 51-52 ºC B.p. Decomposition begins at 160 °C V.p. 0.22 mPa (50 ºC) (Antoine) KOW logP = 3.8 Henry 2.578 ´ 10-4 Pa m3 mol-1 (calc.) S.g./density 1.348 at 25 ºC Solubility In water 4.2 mg/l (25 ºC). Readily soluble in most organic solvents, e.g. xylene, benzene, carbon tetrachloride, dichloromethane, trichloroethylene. In acetone, toluene, ethyl acetate >400, hexane 16.6 (all in g/l, 20 ºC). Stability Hydrolysed by acids and alkalis. Unstable in undiluted form. F.p. 34? °C (Abel-Pensky closed method)
COMMERCIALISATION
History Fungicide reported by F. M. Smit (Meded. Rijksfac. Landbouwwet. Gent, 1969, 34, 763). Introduced by Hoechst AG (now Bayer CropScience). Patents DE 1545790; GB 1145306 Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Inhibits melanin biosynthesis in some species. Prevents development of appressoria by fungal conidia. Mode of action Systemic fungicide with protective and curative action. Absorbed by the leaves and shoots, and translocated acropetally within the plant. Uses Control of Erysiphe, Helminthosporium and Rhynchosporium in cereals, Podosphaera in pome fruit, Erysiphe and Sphaerotheca in cucurbits, Erysiphe in tomatoes, Oidium and Podosphaera in stone fruit, and Uncinula in grapes. Application rates vary from 100-600 g/ha, depending on crop. Phytotoxicity Non-phytotoxic when used as recommended (except to some varieties of grape). Formulation types EC; WP. Selected products: 'Afugan' (Bayer CropScience)
OTHER PRODUCTS
Discontinued products: 'Curamil' * (Hoechst); 'Siganex' * (AgrEvo)
ANALYSIS
Product analysis by hplc (CIPAC Handbook, 1985, 1C, 2206) or by glc. Residues determined by glc (J. Asshauer et al., Anal. Methods Pestic. Plant Growth Regul., 1978, 10, 237; Man. Pestic. Residue Anal., 1987, I, 6, S19; Anal. Methods Residues Pestic., 1988, Part I, M2, M5, M12; A. Ambrus et al., J. Assoc. Off. Anal. Chem., 1981, 64, 773). Details available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 65, 67 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats 151-778 mg/kg (depending on sex and carrier). Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin; slightly irritating to eyes (rabbits). Inhalation LC50 (4 h) for rats 1220 mg/m3 air. NOEL (2 y) for rats 5 mg/kg diet. A 3-generation test on rats showed no effect at 50 mg/kg diet. ADI (JMPR) 0.004 mg/kg b.w. [1992]. Toxicity class WHO (a.i.) II; EPA (formulation) II EC classification Xn; R20/22| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for quail (depending on carrier and sex) 118-480 mg tech./kg. Dietary LC50 (14 d) for mallard ducks c. 340, bobwhite quail c. 300 mg/kg. Fish LC50 (96 h) for carp 2.8-6.1, rainbow trout 0.48-1.14, bluegill sunfish 0.28 mg/l. Daphnia LC50 (48 h) 0.36 mg/l (soft water), 0.63 mg/l (hard water). NOEL 0.18 mg/l in hard and soft water. Algae LC50 (72 h) for Scenedesmus subspicatus 65.5 mg/l. Bees LD50 (24 h, contact) 0.25 mg/bee. Worms LC50 (14 d) for Eisena foetida >1000 mg/kg soil. Other beneficial spp. 'Afugan 30 EC' rated harmful to Poecilus cupreus, Syrphus corollae, Chrysoperla carnea and Aleochara bilineata; harmless to Pardosa amentata (IOBC scheme).
ENVIRONMENTAL FATE
Animals Extensively metabolised and rapidly eliminated in rats, DT50 4-5 h. Excretion mainly in urine; the most important metabolite is ethyl 2-hydroxy-5-methyl-6-pyrazolo[1,5-a]pyrimidine-carboxylate, partly excreted as a sulfate conjugate. Plants Half-life in wheat leaves c. 19 d. Following hydrolysis of the phosphate bond, a b-glucoside of the pyrazolopyrimidine is formed. Soil/Environment Soil degradation occurs by cleavage of the phosphoric acid group, saponification of the carboxylate fraction and further degradation of the heterocyclic ring system, ultimately to CO2. Degradation rates vary with soil type and properties, with no obvious correlation with any single soil characteristic. DT50 10-21 d, DT90 111-235 d (field). Strongly adsorbed to soils, Koc 1332-2670 (calc.). Column studies and leaching models indicate pyrazophos will not leach.
|