|
propoxycarbazone-sodium
Herbicide
HRAC B WSSA 2; sulfonylaminocarbonyltriazolinone
NOMENCLATURE
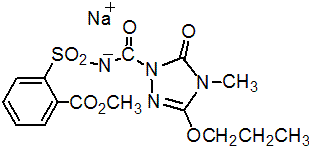
Common name propoxycarbazone (BSI, pa ISO, for the N- acid)
IUPAC name sodium (4,5-dihydro-4-methyl-5-oxo-3-propoxy-1H-1,2,4-triazol-1-ylcarbonyl)(2-methoxycarbonylphenylsulfonyl)azanide (for sodium salt); methyl 2-(4,5-dihydro-4-methyl-5-oxo-3-propoxy-1H-1,2,4-triazol-1-yl)carboxamidosulfonylbenzoate (for N- acid)
Chemical Abstracts name methyl 2-[[[(4,5-dihydro-4-methyl-5-oxo-3-propoxy-1H-1,2,4-triazol-1-yl)carbonyl]amino]sulfonyl]benzoate, sodium salt
Other names procarbazone-sodium (rejected BSI proposal) CAS RN [181274-15-7] sodium salt; [145026-81-9] N- acid Development codes MKH 6561 (Bayer)
PHYSICAL CHEMISTRY
Composition Tech. is 95% pure. Mol. wt. 420.4 M.f. C15H17N4NaO7S Form Colourless, odourless, crystalline powder. M.p. 230-240 °C (decomp.) V.p. <1 ´ 10-5 mPa (20 °C) KOW logP = -0.30 (pH 4), -1.55 (pH 7), -1.59 (pH 9) (all 20 °C) Henry <1 ´ 10-10 Pa m3 mol-1 S.g./density 1.42 (20 °C) Solubility In water 2.9 (pH 4), 42.0 (pH 7), 42.0 (pH 9) (all in g/l, 20 °C). In dichloromethane 1.5, n-heptane, xylene and isopropanol <0.1 (all in g/l, 20 °C). Stability Stable to hydrolysis at pH 4-9 (25 °C). pKa 2.1 (N-acid)
COMMERCIALISATION
History The sodium salt reported by D. Feucht et al., Proc. Br. Crop Prot. Conf. - Weeds, 1999, 1, 53; under development by Bayer AG. Patents EP 00507171 Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential amino acids valine and isoleucine, hence stopping cell division and plant growth. Mode of action Uptake is by leaves and roots. Translocated acropetally and basipetally within both xylem and phloem. Symptoms include stunting, discolouration and necrosis. Uses The sodium salt shows post-emergence control of annual and some perennial grasses, including Bromus spp., Alopecurus myosuroides, Apera spica-venti and Elymus repens, and some broad-leaved weeds, in wheat, rye and triticale, at 30-70 g/ha. Formulation types WG. Selected products: 'Attribut' (sodium salt) (Bayer CropScience); 'Attribute' (sodium salt) (Bayer CropScience); 'Olympus' (sodium salt) (Bayer CropScience)
ANALYSIS
Active substance analysis by hplc (internal standard) - CIPAC No. 655. Methods for the determination of residues are available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >5000 mg/kg. Non-irritating to skin and eyes (rabbits); not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >5030 mg/m3 air. NOEL NOAEL for male rats 43, female rats 49 mg/kg b.w. daily. ADI 0.43 mg/kg b.w. Other Negative in all genotoxicity tests: Salmonella microsome, HPRT, unscheduled DNA, cytogenetics on mammalian cells and mouse micronucleus. No neurotoxicity or oncogenicity. No developmental or reproduction toxicity.
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Dietary LC50 for bobwhite quail >10 566 mg/kg diet. Fish LC50 (96 h) for bluegill sunfish >94.2, rainbow trout >77.2 mg/l. Daphnia EC50 (48 h) >107 mg/l. Algae EC50 (96 h) for green algae 7.36 mg/l. Other aquatic spp. ErC50 (14 h) for Lemna gibba 0.0128 mg/l. Bees LD50 (oral) >319 mg/bee; (contact) >200 mg/bee. Worms LC50 for earthworms >1000 mg/kg dry soil.
ENVIRONMENTAL FATE
Animals Rapid and nearly complete (>88 %) excretion of the administered dose within 48 h, primarily via faeces; 75-89 % unchanged parent compound in urine and faeces. On the basis of metabolism studies in lactating goats, parent compound alone should be regarded as the relevant residue for animals. Plants Based on the results of the metabolism studies in wheat, the unchanged parent compound and its 2-hydroxypropoxy metabolite should be regarded as the relevant residues for plants. Soil/Environment Soil DT50 c. 36 d. Aqueous photolysis DT50 (25 °C) c. 30 d. Field dissipation DT50 c. 9 d.
|