|
primisulfuron-methyl
Herbicide
HRAC B WSSA 2; sulfonylurea
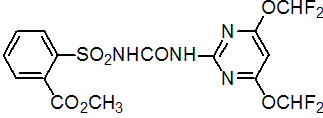
NOMENCLATURE
primisulfuron-methyl
Common name primisulfuron-methyl (BSI, draft E-ISO)
IUPAC name methyl 2-[4,6-bis(difluoromethoxy)pyrimidin-2-ylcarbamoylsulfamoyl]benzoate
Chemical Abstracts name methyl 2-[[[[[4,6-bis(difluoromethoxy)-2-pyrimidinyl]amino]carbonyl]amino]sulfonyl]benzoate
CAS RN [86209-51-0] Development codes CGA 136872 (Ciba-Geigy)
primisulfuron
Common name primisulfuron (BSI, draft E-ISO)
CAS RN [113036-87-6]
PHYSICAL CHEMISTRY
primisulfuron-methyl
Mol. wt. 468.3 M.f. C15H12F4N4O7S Form Fine, white powder. M.p. 194.8-197.4 °C (decomp.) V.p. <5 ´ 10-3 mPa (25 ºC) (OECD 104) KOW logP = 0.2 (25 ºC, pH 7) Henry <0.04 Pa m3 mol-1 (calc.) S.g./density 1.64 (20 ºC) Solubility In water 3.7 (pH 5), 390 (pH 7), 11 000 (pH 8.5) (all in mg/l). In acetone 45 000, toluene 5790, n-octanol 130, n-hexane <1 (all in mg/l, 25 ºC). Stability Stable for at least 3 years at room temperature. Hydrolysis DT50 c. 25 d (pH 5, 25 °C), stable at pH 7 & 9. Stable up to 150 ºC. pKa 3.47
primisulfuron
Mol. wt. 454.3 M.f. C14H10F4N4O7S
COMMERCIALISATION
History Herbicidal activity of primisulfuron-methyl reported by W. Maurer et al. (Proc. 1987 Br. Crop Prot. Conf. - Weeds, 1, 41). Introduced by Ciba-Geigy AG (now Syngenta AG) and first marketed in 1990. Patents EP 84020; US 4478635 Manufacturers Syngenta
APPLICATIONS
primisulfuron-methyl
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential amino acids valine and isoleucine, hence stopping cell division and plant growth. Selectivity derives from rapid metabolism in the crop. Metabolic basis of selectivity in sulfonylureas reviewed (M. K. Koeppe & H. M. Brown, Agro-Food-Industry, 6, 9-14 (1995)). Mode of action Selective systemic herbicide, absorbed through the roots and foliage, with rapid translocation both acropetally and basipetally. Uses Post-emergence control of problem grass weeds, e.g. Sorghum bicolor (shattercane), Sorghum almum, Sorghum halepense (johnsongrass) and Agropyron repens, and many broad-leaved weeds, in maize, at 20-40 g/ha. Phytotoxicity Maize hybrids differ in their sensitivity to primisulfuron-methyl. Formulation types WG; WP. Compatibility Compatible in tank mixtures with atrazine, bromoxynil, cyanazine, dicamba and 2,4-D. Selected products: 'Beacon' (Syngenta); 'Tell' (Syngenta); mixtures: 'Exceed' (+ prosulfuron) (USA) (Syngenta); 'Ring' (+ prosulfuron) (Syngenta)
OTHER PRODUCTS
primisulfuron-methyl
Mixtures: 'Northstar' (+ dicamba-sodium) (Syngenta); 'Spirit' (+ prosulfuron) (Syngenta) Discontinued products: 'Rifle' * (Novartis)
ANALYSIS
Residues determined by hplc. Methods for sulfonylurea residues in crops, soil and water reviewed (A. C. Barefoot et al., Proc. Br. Crop Prot. Conf. - Weeds, 1995, 2, 707).
MAMMALIAN TOXICOLOGY
primisulfuron-methyl
Oral Acute oral LD50 for rats >5050 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2010, mice >2000 mg/kg. Slightly irritating to eyes; non-irritating to skin (rabbits). No skin sensitisation (guinea pigs). Inhalation LC50 (4 h) for rats >4.8 mg/l air. NOEL (2 y) for rats 13 mg/kg b.w. daily; (19 mo) for mice 45 mg/kg b.w. daily; (1 y) for dogs 25 mg/kg b.w. daily. ADI 0.13 mg/kg b.w. Other Non-mutagenic in several tests (e.g., DNA repair and micronucleus). Toxicity class WHO (a.i.) U; EPA (formulation) IV
ECOTOXICOLOGY
primisulfuron-methyl
Birds Oral LD50 for bobwhite quail and mallard ducks >2150 mg/kg. Fish LC50 (96 h) for rainbow trout 29 mg/l. Daphnia LC50 (48 h) 260-480 mg/l. Algae EC50 (7 d) for Selenastrum 24, Anabaena 176, Navicula >227, Skeletonema >222 mg/l. Bees Non-toxic to honeybees; LC50 (48 h, contact) >100 mg/bee. Worms LD50 (14 d) >100 mg/kg soil.
ENVIRONMENTAL FATE
Animals The major metabolic pathway in the rat and other large animals involves hydroxylation of the pyrimidine ring, and partial cleavage of the sulfonylurea bridge to liberate separate phenyl and pyrimidinyl ring moieties. Plants In maize, primisulfuron-methyl is mainly degraded by ring oxidation, followed by sugar conjugation; one major metabolite is 5-hydroxyprimisulfuron-methyl. At harvest time, residues are not detectable (<0.01-0.05 mg/kg) in grain and fodder. Soil/Environment Weakly adsorbed by soil components, but field studies and lysimeter results indicate very low leaching of primisulfuron-methyl. Degradation in the soil is primarily by microbial means; DT50 (field) 4-29 d.
|