|
picoxystrobin
Fungicide
FRAC 11, C3; strobilurin type: methoxyacrylate
NOMENCLATURE
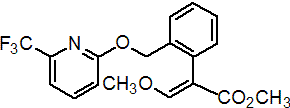
Common name picoxystrobin (BSI, pa ISO)
IUPAC name methyl (E)-3-methoxy-2-[2-(6-trifluoromethyl-2-pyridyloxymethyl)phenyl]acrylate
Chemical Abstracts name methyl (E)-(a)-(methoxymethylene)-2-[[[6-(trifluoromethyl)-2-pyridinyl]oxy]methyl]benzeneacetate
CAS RN [117428-22-5] Development codes ZA 1963 (Zeneca)
PHYSICAL CHEMISTRY
Mol. wt. 367.3 M.f. C18H16F3NO4 Form Colourless powder; (tech. is a solid with creamy colour). M.p. 75 °C V.p. 5.5 ´ 10-3 mPa (20 °C) KOW logP = 3.6 (20 °C) Henry 6.5 ´ 10-4 Pa m3 mol-1 (calc.) S.g./density 1.4 (20 °C) Solubility In water 3.1 mg/l (20 °C)
COMMERCIALISATION
History Reported by J. R. Godwin et al. (Proc. BCPC Conf. - Pests Dis., 2000, 2, 533). Developed and introduced by Zeneca Agrochemicals (now Syngenta AG) and first registered in 2001. Manufacturers Syngenta
APPLICATIONS
Biochemistry Inhibits mitochondrial respiration by blocking electron transfer at the Qo centre of cytochrome bc1. Mode of action Preventative and curative fungicide with unique distribution properties including systemic (acropetal) and translaminar movement, diffusion in leaf waxes and molecular redistribution in air. Uses For broad spectrum disease control, including Mycosphaerella graminicola, Phaeosphaeria nodorum, Puccinia recondita (brown rust), Helminthosporium tritici-repentis (tan spot) and Blumeria graminis f.sp. tritici (strobilurin-sensitive powdery mildew) in wheat; Helminthosporium teres (net blotch), Rhynchosporium secalis, Puccinia hordei (brown rust), Erysiphe graminis f.sp. hordei (strobilurin-sensitive powdery mildew) in barley; Puccinia coronata and Helminthosporium avenae, in oats; and Puccinia recondita, Rhynchosporium secalis in rye. Application typically 250 g/ha. Formulation types SC. Selected products: 'Acanto' (Syngenta)
OTHER PRODUCTS
Mixtures: 'Acanto Dos' (+ hexaconazole) (cereals) (Syngenta); 'Acanto Duo Pack' (+ propiconazole+ fenpropidin) (Syngenta)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin and eyes (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 for rats >2.12 mg/l NOEL NOAEL (subchronic, dogs) 4.3 mg/kg b.w. daily. ADI 0.04 mg/kg b.w. Other Non-genotoxic; no developmental toxicity potential (rats and rabbits); no reprotoxicity potential (rats); no carcinogenic potential (rats and mice).
ECOTOXICOLOGY
Birds LD50 for bobwhite quail >5200 mg/kg. NOEC (21 w) for mallard ducks 1350 mg/kg. Fish LC50 (96 h) (two species) 65-75 mg/l. Daphnia EC50 (48 h) 18 mg/l. Algae EC50 (72 h) for Selenastrum capricornutum 56 mg/l. Other aquatic spp. EC50 for Chironomus riparius 19 mg/kg (28 d, dosed to sediment), 140 mg/l (25 d, dosed to water) Bees LD50 (48 h, contact and oral) >200 mg/bee Worms LC50 (14 d) for Eisenia foetida 6.7 mg/kg soil. Other beneficial spp. Lab. and field tests with 6 species of non-target arthropods indicate low risk to populations. LR50 (7 d) for Typhlodromus pyri 12.6 g/ha; LR50 (2 d) for Aphidius rhopalosiphi 280 g/ha.
ENVIRONMENTAL FATE
Animals In rats, well absorbed extensively metabolised and rapidly eliminated. Does not accumulate in meat or milk. Plants Residues in cereals are low (<0.01-0.20 mg/kg). Soil/Environment Rapidly degraded in soils, with CO2 as the major product; lab. DT50 (aerobic) 19-33 d; field dissipation DT50 3-35 d. Not mobile in soil under field conditions; Koc 790-1200. Rapid dissipation in water indicates no chronic issues for aquatic organisms; water phase DT50 7-15 d (lab. and outdoor water sediment systems).
|