|
picolinafen
Herbicide
HRAC F1 WSSA 12; pyridinecarboxamide
NOMENCLATURE
Common name picolinafen (BSI, pa ISO)
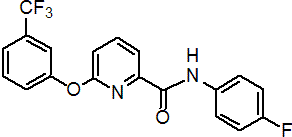
IUPAC name 4'-fluoro-6-(a,a,a-trifluoro-m-tolyloxy)pyridine-2-carboxanilide
Chemical Abstracts name N-(4-fluorophenyl)-6-[3-(trifluoromethyl)phenoxy]-2-pyridinecarboxamide
CAS RN [137641-05-5] Development codes AC 900001; CL 900001 (both Cyanamid); WL 161616 (Shell); BAS 700 H (BASF)
PHYSICAL CHEMISTRY
Mol. wt. 376.3 M.f. C19H12F4N2O2 Form White, finely crystalline solid, with a musty, phenolic odour. M.p. 107.2-107.6 °C B.p. Decomp. >230 °C V.p. 1.66 ´ 10-7 mPa (est., 20 °C) KOW logP = 5.37 Henry 1.6 ´ 10-3 Pa m3 mol-1 (calc.) S.g./density 1.45 Solubility In water 3.9 ´ 10-5 g/l (distilled water, 20 °C); 4.7 ´ 10-5 g/l (pH 7, 20 °C). In acetone 55.7, dichloromethane 76.4, ethyl acetate 46.4, methanol 3.04 (all in g/100 ml). Stability Stable to hydrolysis at pH 4, 7 and 9 over 5 d (50 °C). Photodegradation DT50 24.8 d (pH 5), 31.4 d (pH 7), 22.6 d (pH 9). F.p. >180 °C
COMMERCIALISATION
History Discovered by Shell International Research. Acquired by American Cyanamid Company (now BASF AG). Reported by R. H. White et al., Proc. Br. Crop Prot. Conf. - Weeds, 1999, 1, 47. Introduced by BASF in 2001. Manufacturers BASF
APPLICATIONS
Biochemistry Inhibitor of phytoene desaturase, leading to blocking of carotenoid biosynthesis. Selectivity appears to be due to differential uptake and translocation. Mode of action Post-emergence herbicide with rapid foliar uptake in susceptible species. Little or no root uptake. Causes whitening of leaves of susceptible weeds. Uses Cereal herbicide, under development for post-emergence control of broad-leaved weeds, such as Galium, Viola, Lamium and Veronica spp., at 0.05 up to 0.10 kg/ha. To be marketed in mixture with other cereal herbicides, such as pendimethalin, isoproturon, MCPA; also to be developed for use in lupins and pasture. Formulation types EC; SC; WG. Selected products: 'Pico' (BASF)
OTHER PRODUCTS
'Sniper' (BASF) mixtures: 'Celtic' (+ pendimethalin) (BASF); 'Paragon' (+ MCPA-2-ethylhexyl) (BASF); 'PicoPro' (+ pendimethalin) (BASF)
ANALYSIS
Details available from BASF.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male and female rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for male and female rats >4000 mg/kg. Non-irritating to skin and eyes (rabbits); not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for male and female rats >5.9 mg/l. NOEL NOAEL (1 y) for dogs 1.5 mg/kg b.w. daily; (2 y) for rats 2.7 mg/kg b.w. daily. ADI 0.015 mg/kg b.w. Other Negative in Ames, HGPRT/CHO, micronucleus and in vitro cytogenetics tests.
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail and mallard ducks >2250 mg/kg. Dietary LC50 for bobwhite quail and mallard ducks >5314 ppm in diet. Fish LC50 (96 h) for rainbow trout 0.281, bluegill sunfish >0.57 mg/l. Daphnia EC50 (48 h) 0.612 mg/l. Algae EC50 (72 h) for Anabaena 0.12 mg/l, for Selenastrum capricornutum 0.18 mg/l. Other aquatic spp. EC50 for Lemna 0.057 mg/l. Bees LD50 (oral and dermal) >200 mg/bee. Worms LC50 (14 d) for earthworms >1000 mg/kg. Other beneficial spp. Harmless to Typhlodromus, Poecilus, Aphidius, and Pardosa.
ENVIRONMENTAL FATE
Animals Rapidly metabolised by hydrolytic cleavage of the amide bond, to yield the substituted picolinic acid and p-fluoroaniline. Plants Slowly metabolised in wheat with little translocation of parent or metabolites. Metabolism proceeds via cleavage of the amide bond. Soil/Environment Hydrolytically stable, but photochemically degraded, DT50 23-31 d. Average field DT50 1 mo, DT90 <4 mo. Strongly bound to soil, Koc (four soil types) 15 100-31 800, Kd 248-764.
|