|
phenmedipham
Herbicide
HRAC C1 WSSA 5; phenyl carbamate herbicide
NOMENCLATURE
Common name phenmedipham (BSI, E-ISO, ANSI, WSSA, JMAF); phenmédiphame ((m) F-ISO)
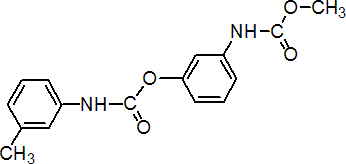
IUPAC name methyl 3-(3-methylcarbaniloyloxy)carbanilate; 3-methoxycarbonylaminophenyl 3'-methylcarbanilate
Chemical Abstracts name 3-[(methoxycarbonyl)amino]phenyl (3-methylphenyl)carbamate
Other names PMP CAS RN [13684-63-4] Development codes SN 38 584 (Schering); ZK 15320; EP-452
PHYSICAL CHEMISTRY
Composition Tech. grade is >97% pure. Mol. wt. 300.3 M.f. C16H16N2O4 Form Colourless crystals. M.p. 143-144 ºC; (tech., 140-144 ºC) V.p. 1.33 ´ 10-6 mPa (25 ºC) KOW logP = 3.59 (pH 3.9) Henry 5 ´ 10-8 Pa m3 mol-1 (calc.) S.g./density 0.34-0.54 (20 ºC) Solubility In water 4.7 mg/l (room temperature). Soluble in polar organic solvents. In acetone, cyclohexanone c. 200, methanol c. 50, chloroform 20, benzene 2.5, hexane c. 0.5, dichloromethane 16.7, ethyl acetate 56.3, toluene c. 0.97, 2,2,4-trimethylpentane 1.16 (all in g/l, 20 ºC). Stability Stable up to 200 ºC. Very stable in acidic media, but hydrolysed in neutral and alkaline media; DT50 (22 ºC) 50 d (pH 5), 14.5 h (pH 7), 10 min (pH 9). In solution (pH 3.8) irradiated at 280 nm, DT50 9.7 d. pKa <0.1
COMMERCIALISATION
History Herbicide reported by F. Arndt & C. Kötter (Abstr. Int. Congr. Plant Prot., 6th, Vienna, 1967, p. 433). Introduced by Schering AG (now Bayer CropScience). Patents GB 1127050 Manufacturers Bayer CropScience; Griffin; Sharda; Synthesia; United Phosphorus Ltd
APPLICATIONS
Biochemistry Photosynthetic electron transport inhibitor at the photosystem II receptor site. Mode of action Selective systemic herbicide, absorbed through the leaves, with translocation primarily in the apoplast. Uses Used post-emergence, at 1 kg/ha, in beet crops, especially sugar beet, after the emergence of most broad-leaved weeds and before they develop more than 2-4 true leaves; also used on strawberries, spinach, peas, mangold and red beet. Phytotoxicity Non-phytotoxic to beet crops. Formulation types EC; SC; oil-SC; SL; WG. Compatibility Incompatible with alkaline substances. Selected products: 'Alegro' (Bayer CropScience); 'Beetup' (United Phosphorus Ltd); 'Beta' (Stefes, Tripart); 'Betanal' (Bayer CropScience); 'Betapost' (Agriphar); 'Betasana' (United Phosphorus Ltd); 'Herbasan' (Nufarm UK); 'Kemifam' (Bayer CropScience); 'Kontakt' (Feinchemie Schwebda); 'Mandolin' (Griffin); 'Punter' (Barclay); 'Spin-aid' (Bayer CropScience); 'Vangard' (FCC); mixtures: 'Betanal Tandem' (+ ethofumesate) (Bayer CropScience)
OTHER PRODUCTS
'Betanal Flo' (Bayer CropScience); 'Betosip' (Sipcam); 'Crotale' (Stefes); 'Dancer' (Sipcam); 'MSS Protrum G' (Nufarm UK) mixtures: 'Agricola Lens' (+ lenacil) (Agricola); 'Beetup Pro' (+ desmedipham+ ethofumesate) (United Phosphorus Ltd); 'Beetup Trio' (+ desmedipham+ ethofumesate) (United Phosphorus Ltd); 'Betamix Progress' (+ desmedipham+ ethofumesate) (Bayer CropScience); 'Betamix' (+ desmedipham) (Bayer CropScience); 'Betanal Compact' (+ desmedipham) (Bayer CropScience); 'Betanal Progress OF' (+ desmedipham+ ethofumesate) (Bayer CropScience); 'Betanal Quattro' (+ desmedipham+ ethofumesate+ metamitron) (Bayer CropScience); 'Betanal Trio' (+ ethofumesate+ metamitron) (Bayer CropScience); 'Betasana Trio' (+ desmedipham+ ethofumesate) (United Phosphorus Ltd); 'Betosip Combi' (+ ethofumesate) (Sipcam); 'Bettix Triple' (+ desmedipham+ ethofumesate+ metamitron) (United Phosphorus Ltd); 'Contatto' (+ desmedipham+ ethofumesate) (Feinchemie Schwebda); 'Goalpost' (+ ethofumesate) (Barclay); 'Goldpost' (+ ethofumesate) (Barclay); 'Kontakt Twin' (+ ethofumesate) (Feinchemie Schwebda); 'MagicTandem' (+ ethofumesate) (Stefes); 'Powertwin' (+ ethofumesate) (Feinchemie Schwebda); 'Progress' (+ desmedipham+ ethofumesate) (Bayer CropScience); 'Safen' (+ desmedipham) (Azot); 'Saherb' (+ trifluralin+ desmedipham+ ethofumesate) (Azot); 'Synbetan Duo' (+ desmedipham) (Synthesia); 'Synbetan Mix' (+ desmedipham) (Synthesia); 'Synbetan P Forte' (+ ethofumesate) (Synthesia); 'Twin' (+ ethofumesate) (Feinchemie Schwebda, Headland) Discontinued products: 'Betaflow' * (AgrEvo); 'Betanal E' * (Aventis); 'Atlas Protrum K' * (Atlas); 'Beetomax' * (Fine); 'Betalion' * (Portman); 'Betaren' * (PBI); 'Cirrus' * (Quadrangle); 'Dephend' * (Headland); 'Fender' * (ISK Biosciences); 'Forte' * (Stefes); 'Goliath' * (ABM); 'Medipham' * (Stefes); 'MSS Betaren' * (Mirfield); 'Pistol' * (ABM); 'Protrum K' * (Nufarm Whyte); 'Suplex' * (UCP) mixtures: 'DUK 880' * (+ lenacil) (DuPont); 'Medimat 2' * (+ ethofumesate) (Stefes); 'Stefes 880' * (+ lenacil) (Stefes); 'Tandem' * (+ ethofumesate) (Stefes)
ANALYSIS
Product analysis by titration (CIPAC Handbook, 1985, 1C, 2181) or by hplc (ibid., 2184); method available from Bayer CropScience. Residues by glc (K. Kossmann & N. A. Jenny, Anal. Methods Pestic. Plant Growth Regul., 1973, 7, 611); in soil by hplc or by hydrolysis to m-toluidine, derivatives of which are determined by glc with ECD or by colorimetry (K. Kossmann, Weed Res., 1970, 10, 340). Details available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats and mice >8000, guinea pigs and dogs >4000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits 1000, rats 2500 mg/kg. Not a skin sensitiser. Inhalation LC50 (4 h) for rats >7.0 mg/l. NOEL (2 y) for rats 100, dogs 1000 mg/kg; (90 d) for rats and dogs 200 mg/kg diet. ADI 0.03 mg/kg. Other Acute i.p. LD50 for rats >5000 mg/kg. Toxicity class WHO (a.i.) U; EPA (formulation) IV
ECOTOXICOLOGY
Birds Acute oral LD50 for chickens >2500, mallard ducks >2100 mg/kg. Dietary LC50 (8 d) for mallard ducks and bobwhite quail >6000 mg/kg diet. Fish LC50 (96 h) for rainbow trout 1.4-3.0, bluegill sunfish 3.98 mg/l. LC50 (96 h) for harlequin fish 16.5 mg/l (15.9% EC formulation). Daphnia LC50 (72 h) 3.8 mg/l. Algae IC50 (96 h) 0.13 mg/l. Bees Not toxic to bees; LD50 (oral) >23 mg/bee; (contact) 50 mg/bee. Worms EC50 (14 d) >156 mg/kg soil.
ENVIRONMENTAL FATE
Animals In mammals, following oral administration, 99% is excreted within 72 hours, mainly in the urine. Hydrolysis to methyl N-(3-hydroxyphenyl)carbamate and conjugation to glucuronides and ethereal sulfates are the major steps in metabolism. Plants Methyl N-(3-hydroxyphenyl)carbamate is the major metabolite in plants. Soil/Environment DT50 in soil c. 25 d, DT90 c. 108 d. Metabolites include methyl N-(3-hydroxyphenyl)carbamate and m-aminophenol, and subsequently complexes with soil components (R. Senawana et al., Bull. Environ. Contam. Toxicol., 1971, 6, 322-327). Phenmedipham does not accumulate in soil, nor is there any relevant uptake by following crops. Due to its favourable physico-chemical parameters, no risk of groundwater contamination is to be expected. Koc 2400.
|