|
permethrin
Insecticide
IRAC 3; pyrethroid
NOMENCLATURE
Common name permethrin (BSI, E-ISO, ANSI, ESA, BAN); perméthrine (F-ISO); no name (Eire, Bangladesh)
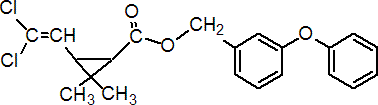
IUPAC name 3-phenoxybenzyl (1RS,3RS;1RS,3SR)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Roth: 3-phenoxybenzyl (1RS)-cis-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Chemical Abstracts name (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
CAS RN [52645-53-1],(formerly [57608-04-5] and [63364-00-1]) EEC no. 258-067-9 Development codes NRDC 143; FMC 33 297; PP557 (ICI); WL 43 479 (Shell); LE 79-519 (Rhône-Poulenc) Official codes OMS 1821
PHYSICAL CHEMISTRY
Mol. wt. 391.3 M.f. C21H20Cl2O3 Form Tech. is a yellow-brown to brown liquid, which sometimes tends to crystallise partly at room temperature. M.p. 34-35 ºC; cis- isomers 63-65 ºC; trans- 44-47 ºC B.p. 200 ºC/0.1 mmHg; >290 ºC/760 mmHg V.p. cis- 0.0025 mPa; trans- 0.0015 mPa (both 20 ºC) (D. Wells et al., Pestic. Sci., 1986, 17,473) KOW logP = 6.1 (20 ºC) S.g./density 1.29 (20 °C) Solubility In water 6 ´ 10-3 mg/l (pH 7, 20 ºC). In xylene, hexane >1000, methanol 258 (all in g/kg, 25 ºC). Stability Stable to heat (³2 y at 50 ºC), more stable in acidic than alkaline media with optimum stability c. pH 4; DT50 50 d (pH 9), stable (pH 5, 7) (all 25 °C). Some photochemical degradation observed in laboratory studies, but field data indicate this does not adversely affect biological performance. F.p. >100 ºC ('Perigen')
COMMERCIALISATION
History Insecticide reported by M. Elliott et al. (Proc. Br. Insectic. Fungic. Conf., 7th, 1973, 2, 721; Nature (London), 1973, 246, 169). Developed by FMC Corp., ICI Agrochemicals (now Syngenta AG), Mitchell Cotts Chemicals, Penick Corp., Shell International Chemical Co., Ltd (now BASF AG), Sumitomo Chemical Co., Ltd and the Wellcome Foundation. Patents GB 1413491 to NRDC Manufacturers Agro-Chemie; Agrochem; BASF; Bharat; Dow AgroSciences; Fertiagro; FMC; Jiangsu Yangnong; Meghmani; Mitchell Cotts; Mitsu; Parry; RPG; Sharda; Sumitomo; Sundat; Syngenta; Tagros; United Phosphorus
APPLICATIONS
Biochemistry Acts on the nervous system of insects, disturbs the function of neurons by interaction with the sodium channel. Mode of action Non-systemic insecticide with contact and stomach action, having a slight repellent effect. Uses A contact insecticide effective against a broad range of pests. It controls leaf- and fruit-eating Lepidoptera and Coleoptera in cotton, at 100-150 g/ha, in fruit, at 25-50 g/ha, in tobacco, vines and other crops, at 50-200 g/ha, and in vegetables, at 40-70 g/ha. It has good residual activity on treated plants. It is effective against a wide range of animal ectoparasites, provides >60 d residual control of biting flies in animal housing, at 200 mg a.i. (as EC)/m2 wall or 30 mg a.i. (as WP)/m2 wall, and is effective as a wool preservative, at 200 mg/kg wool. It provides >120 d control of Blattodea, Diptera, Hymenoptera and other crawling insects, at 100 mg a.i. (as WP)/m2, also flying insects. Phytotoxicity Non-phytotoxic when used as directed (except that some ornamentals may be injured). Formulation types DP; EC; UL; WG; WP; Fumigant; Aerosol. Compatibility Mixing with calcium nitrate is not recommended. Selected products: 'Ambush' (crop protection use) (Syngenta, Amvac); 'Dragnet' (termiticide) (FMC); 'Dragon' (public health) (Syngenta); 'Eksmin' (Sumitomo); 'Outflank' (veterinary use) (BASF); 'Pounce' (FMC); 'Talcord' (agronomic use) (BASF); 'Agniban' (Devidayal); 'Assithrin' (Frunol); 'Cliper' (Cequisa); 'Mikrem' (micro-encapsulated) (Kemio); 'Onesol' (aerosol) (Kemio); 'Perkill' (United Phosphorus); 'Permetiol' (Agriphar); 'Permit' (Agricultura Nacional, Sanonda); 'Permost' (Hockley); 'Persect' (public health) (Vapco); 'Sanathrin' (Dow AgroSciences); mixtures: 'Chinethrin' (+ piperonyl butoxide+ tetramethrin) (Agro-Chemie)
OTHER PRODUCTS
'Adion' (Sumitomo); 'Imperator' (Syngenta); 'Permasect' (Mitchell Cotts, Parry); 'Stockade' (veterinary use) (BASF); 'Corsair' (Bayer CropScience); 'Kemetrin' (Kemio); 'Kerp' (Kemio); 'Perigen' (Bayer CropScience); 'Permeth' (Agrochem); 'Pertec' (Tecomag); 'Pramex' (Valent Biosciences); 'Qamlin' (Bayer CropScience); 'Tengard' (United Phosphorus Inc); 'Tornade' (Bayer CropScience) mixtures: 'A-9497b' (+ codlemone) (IPM Technologies); 'Allevol' (+ piperonyl butoxide+ bioallethrin) (Kemio); 'Aqua Reslin' (+ bioallethrin S-cyclopentenyl isomer) (Bayer CropScience); 'Bipol' (+ diazinon) (Kemio); 'Coopex' (+ bioallethrin S-cyclopentenyl isomer) (Kwizda); 'Duracide P' (+ piperonyl butoxide+ tetramethrin) (Endura); 'Metrovol' (+ piperonyl butoxide+ bioallethrin) (Kemio); 'Neopybuthrin' (+ bioallethrin+ bioallethrin S-cyclopentenyl isomer) (Bayer CropScience); 'PB' (+ tetramethrin) (Trithin); 'Permanone' (+ piperonyl butoxide) (Bayer CropScience, Valent Biosciences); 'Phinco - T 22' (+ piperonyl butoxide+ tetramethrin) (Vapco); 'Sirene-CM' (+ codlemone) (IPM Technologies); 'Sirene-PBW' (+ gossyplure) (IPM Technologies) Discontinued products: 'Kafil' * (crop protection use) (Zeneca); 'Prelude' * (Zeneca); 'Darmycel Agarifume Smoke Generator' * (Sylvan); 'Permit' * (PBI) mixtures: 'Ars Red' * (+ metoxadiazone) (Sumitomo); 'Valsan' * (+ metoxadiazone) (Sumitomo); 'Wiperjet' * (+ metoxadiazone) (Sumitomo); 'Combinex' * (+ thiram) (Fargro); 'Turbair Grain Store Insecticide' * (+ resmethrin+ fenitrothion) (Graincare)
ANALYSIS
Product analysis of pyrethroids reviewed by E. Papadopoulou-Mourkidou in Comp. Analyt. Profiles. By glc with FID (CIPAC Handbook, 1985, 1C, 2172; AOAC Methods, 17th Ed., 986.03; H. Swaine & M. J. Tandy, Anal. Methods Pestic. Plant Growth Regul., 1984, 13, 103). Identity by glc, hplc or spectophotometrically (CIPAC Handbook, 1994, F, 404). Residues determined by glc with ECD (idem, ibid.; AOAC Methods, 17th Ed., 998.01; A. Ambrus et al., J. Assoc. Off. Anal. Chem., 1981, 64, 733; Man. Pesticide Residue Anal., 1987, I, 6, S19; Anal. Methods Residues Pestic., 1988, Part I, M11). Permethrin isomers in drinking water by glc with ECD (AOAC Methods, 17th Ed., 990.06).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 86, 88 (see part 2 of the Bibliography). See also A. J. Gray & D. M. Soderlund, Chapt. 5 in "Insecticides". IARC ref. 53 class 3 Oral Oral LD50 values of permethrin depend on such factors as: carrier, cis/trans ratio of the sample, the test species, its sex, age and degree of fasting; values reported sometimes differ markedly. Values for a cis/trans ratio of c. 40:60 are: for rats 430-4000, mice 540-2690 mg/kg; with a 20:80 ratio, the LD50 is c. 6000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2500, rabbits >2000 mg/kg. Mild eye and skin irritant (rabbits). Moderate skin sensitiser. Inhalation LC50 (3 h) for mice and rats >685 mg/m3 air; (separate study gives >13800 mg/m3). NOEL In 2 y feeding trials, rats receiving 100 mg/kg diet showed no ill-effects. ADI (JMPR) 0.05 mg/kg b.w. (for tech., with cis/trans ratios 25:75 to 40:60) [1999]. Water GV 20 mg/l (based on ADI). Other No mutagenic, teratogenic, or carcinogenic activity. Toxicity class WHO (a.i.) II; EPA (formulation) II ('Ambush'); III ('Outflank') EC classification Xn; R22
ECOTOXICOLOGY
Birds Typical oral LD50 values for a cis/trans ratio of c. 40:60 are: for chickens >3000, mallard ducks >9800, Japanese quail >13 500 mg/kg. Fish LC50 (96 h) for rainbow trout 2.5 mg/l; (48 h) for rainbow trout 5.4, bluegill sunfish 1.8 mg/l. Daphnia LC50 (48 h) 0.6 mg/l. Bees Toxic to bees. LD50 (24 h) (oral) 0.098 mg/bee; (topical) 0.029 mg/bee.
ENVIRONMENTAL FATE
EHC 94 (WHO, 1990). EHC 94 concludes that permethrin is not likely to be a hazard to the environment when used as recommended. Animals In mammals, there is hydrolysis of the ester bond, hydroxylation, and elimination as the glucoside conjugate (M. Elliott et al., J. Agric. Food Chem., 1976, 24, 270; L. C. Gaughan et al., ibid., 1978, 26, 613). Soil/Environment In soil and water, degradation is rapid. DT50 in soil <38 d (pH 4.2-7.7, o.m. 1.3-51.3%) (R. L. Holmstead et. al., J. Agric. Food Chem., 1978, 26, 590).
|