|
pentoxazone
Herbicide
HRAC E WSSA 14; oxazolidinedione
NOMENCLATURE
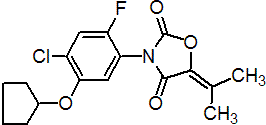
Common name pentoxazone (BSI, pa ISO)
IUPAC name 3-(4-chloro-5-cyclopentyloxy-2-fluorophenyl)-5-isopropylidene-1,3-oxazolidine-2,4-dione
Chemical Abstracts name 3-[4-chloro-5-(cyclopentyloxy)-2-fluorophenyl]-5-(1-methylethylidene)-2,4-oxazolidinedione
CAS RN [110956-75-7] Development codes KPP-314 (Kaken)
PHYSICAL CHEMISTRY
Mol. wt. 353.8 M.f. C17H17ClFNO4 Form Colourless, odourless, crystalline powder. M.p. 104 °C V.p. <1.11 ´ 10-2 mPa (25 °C) KOW logP = 4.66 (25 °C) Henry <1.82 ´ 10-2 Pa m3 mol-1 (calc.) S.g./density 1.418 (25 °C) Solubility In water 0.216 mg/l (25 °C). In methanol 24.8, hexane 5.10 (both in g/l). Stability Stable to heat, light and acid; unstable in alkali.
COMMERCIALISATION
History Discovered in 1986 by Sagami Chemical Research Center, and developed by Kaken Pharmaceutical Co., Ltd. First registered in Japan in 1997. Manufacturers Kaken
APPLICATIONS
Biochemistry Protoporphyrinogen oxidase inhibitor. This enzyme catalyses the conversion of protoporphyrinogen IX to protoporphyrin IX in plant chlorophyll biosynthesis. Uses Pre- and post-emergence control of Echinochloa spp. and Monochoria vaginalis in rice, at 150-450 g/ha. Formulation types EW; GR; SC; WG. Selected products: 'Wechser' (Kaken); mixtures: 'Kusabue' (+ cumyluron) (Marubeni, Yashima, Nissan, Kaken); 'Shokinie' (+ bromobutide) (Kumiai); 'Topgun' (+ pyriminobac-methyl+ bensulfuron-methyl+ bromobutide) (Kumiai)
OTHER PRODUCTS
Mixtures: 'Kusa Punch' (+ daimuron) (Kaken); 'Sakidori' (+ butachlor) (Kaken, Kumiai); 'Staabo' (+ pyrazosulfuron-ethyl) (Kaken, Nissan); 'Tema Cut' (+ daimuron) (Kaken); 'The-One' (+ daimuron+ imazosulfuron) (Kaken, Sumitomo Chemical Takeda); 'Focus Shot' (+ benzobicyclon) (SDS Biotech KK, Kaken); 'Utopia' (+ cyclosulfamuron) (BASF, Kaken)
ANALYSIS
By hplc.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male and female rats, and male and female mice >5000 mg/kg. Skin and eye Acute percutaneous LD50 for male and female rats >2000 mg/kg. Not a skin or eye irritant (rabbits); not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for male and female rats >5100 mg/m3. NOEL for male rats 6.92, female rats 43.8 mg/kg b.w. daily; for male mice 250.9, female mice 190.6 mg/kg b.w. daily; for male dogs 23.1, female dogs 25.2 mg/kg b.w. daily. Other Not carcinogenic or teratogenic; negative in Ames, DNA repair and micronucleus tests.
ECOTOXICOLOGY
Birds Acute oral LD50 for male and female bobwhite quail >2250 mg/kg. Fish LC50 (96 h) for carp 21.4 ppm. Daphnia LC50 (24 h) >38.8 ppm. Algae EC50 (72 h) for Selenastrum capricornutum 1.31 ppb. Bees LC50 (oral) >458.5 ppm; (contact) 98.7 mg/bee. Worms LC50 (14 d) >851 ppm. Other beneficial spp. LC50 (96 h) for silkworms >458.5 ppm.
ENVIRONMENTAL FATE
Animals Male and female rats excrete >95% of administered dose, mainly via the faeces, within 48 h. Soil/Environment In soil, DT50 <29 d (two types of flooded soil, 28 °C). In water, DT50 1.4 d (pH 8.0, 20 °C). Soil Koc 3160.
|