|
oxydemeton-methyl
Insecticide
IRAC 1B; organophosphate
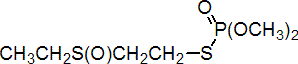
NOMENCLATURE
Common name oxydemeton-methyl (BSI, E-ISO, (m) F-ISO); oxydemetonmethyl (ESA); oxydemeton-metyl (USSR); metilmerkaptofosoksid* (former USSR name)
IUPAC name S-2-ethylsulfinylethyl O,O-dimethyl phosphorothioate
Chemical Abstracts name S-[2-(ethylsulfinyl)ethyl] O,O-dimethyl phosphorothioate
CAS RN [301-12-2] EEC no. 206-110-7 Development codes Bayer 21 097; R 2170 (Bayer) Official codes ENT 24 964
PHYSICAL CHEMISTRY
Mol. wt. 246.3 M.f. C6H15O4PS2 Form Colourless liquid. M.p. <-20 ºC B.p. 106 ºC/0.01 mmHg V.p. 3.8 mPa (20 ºC) KOW logP = -0.74 (21 °C) Henry <<1 ´ 10-5 Pa m3 mol-1 (calc.) S.g./density 1.289 (20 ºC) Solubility Completely miscible with water. Soluble in common organic solvents, except in petroleum ether. Stability Relatively slowly hydrolysed in acidic media, but rapidly hydrolysed in alkaline media; DT50 (est.) 107 d (pH 4), 46 d (pH 7), 2 d (pH 9) (22 ºC). F.p. 113 °C
COMMERCIALISATION
History Insecticide reported by G. Schrader (Die Entwicklung neuer insektizider Phosphorsäure-Ester). Introduced by Bayer AG. Patents DE 947368; US 2963505 Manufacturers Aimco; Bayer India
APPLICATIONS
Biochemistry Cholinesterase inhibitor. Mode of action Systemic insecticide with contact and stomach action. Has quick knockdown effect. Uses Control of aphids, sawflies, suckers, and other sucking insects on fruit, vines, vegetables, cereals and ornamentals, at rates of 0.3-0.8 kg/ha.. Phytotoxicity Some ornamentals may be injured, more especially in combination with other pesticides. Formulation types EC; SL. Compatibility Incompatible with alkaline materials. Selected products: 'Aimcosystox' (Aimco); 'Devisystox' (Devidayal); 'Dhanusystox' (Dhanuka); 'Metasystox R' (Bayer CropScience, Gowan, Makhteshim-Agan)
OTHER PRODUCTS
'Anthonox' (Makhteshim-Agan, Bayer CropScience); 'Harpoon' (Florida Silvics); 'MSR' (Gowan) Discontinued products: 'Metasystemox R' * (Bayer)
ANALYSIS
Product analysis by hplc (AOAC Methods, 17th Ed., 991.05; CIPAC Handbook, 1992, E, 163-165), by tlc followed by oxidation to phosphoric acid which is measured by standard colorimetric methods (CIPAC Handbook, 1983, 1B, 1871), or by reduction of the sulfoxide group by titanium(III) sulfate and titration of the excess (details from Gowan Co.). Residues determined, after oxidation to the corresponding sulfone (demeton-S-methylsulphon), by glc with FID (K. Wagner & J. S. Thornton, Pflanzenschutz-Nachr. (Engl. Ed.), 1977, 30, 1; Anal. Methods Pestic. Plant Growth Regul., 1972, 6, 432). Methods for the determination of residues are available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 56, 58 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats c. 50 mg/kg. Skin and eye Acute percutaneous LD50 for rats c. 130 mg/kg. Mild skin irritant; eye irritant (rabbits; 50% in MIBK). Inhalation LC50 (4 h) for female rats 427 mg/m3 (50% in MIBK). NOEL (2 y) for rats 1, mice 30 mg/kg diet; (1 y) for dogs 0.25 mg/kg b.w. ADI (JMPR) 0.0003 mg/kg for sum of demeton-S-methyl, demeton-S-methylsulphon and oxydemeton-methyl [1989]. Other Not a primary embryotoxin, not a mutagen. Toxicity class WHO (a.i.) Ib; EPA (formulation) I EC classification T; R24/25| N; R50
ECOTOXICOLOGY
Birds LD50 for bobwhite quail 34-37 mg/kg. LC50 (5 d) for mallard ducks >5000, bobwhite quail 434 mg/kg diet. Fish LC50 (96 h) for rainbow trout 17, golden orfe 447.3, bluegill sunfish 1.9 mg/l. Daphnia LC50 (48 h) 0.19 mg/l. Algae ErC50 for Scenedesmus subspicatus 49 mg/l. Bees Toxic to bees. Worms LC50 for Eisenia foetida 115 mg/kg dry soil.
ENVIRONMENTAL FATE
EHC 63 (WHO, 1986; a general review of organophosphorus insecticides). Animals Elimination is very quick; almost 99% is excreted within 48 h in the urine. Plants Oxydemeton-methyl is very quickly metabolised in sugar beet. Besides oxidation to biologically-active demeton-S-methylsulphon, the main metabolic reactions are hydrolysis and subsequent dimerisation, as well as the formation of conjugates. Soil/Environment Degradation in different soils is extremely rapid. The main metabolic routes involve oxidation of the sulfoxide to a sulfone group, and oxidative and hydrolytic cleavage of the side-chain, with the formation of dimethylphosphoric acid and phosphoric acid.
|