|
oxaziclomefone
Herbicide
HRAC Z
NOMENCLATURE
Common name oxaziclomefone (BSI, pa ISO)
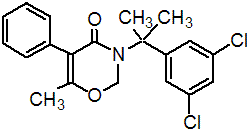
IUPAC name 3-[1-(3,5-dichlorophenyl)-1-methylethyl]-3,4-dihydro-6-methyl-5-phenyl-2H-1,3-oxazin-4-one
Chemical Abstracts name 3-[1-(3,5-dichlorophenyl)-1-methylethyl]-2,3-dihydro-6-methyl-5-phenyl-4H-1,3-oxazin-4-one
CAS RN [153197-14-9] Development codes MY-100
PHYSICAL CHEMISTRY
Mol. wt. 376.3 M.f. C20H19Cl2NO2 Form White crystals. M.p. 149.5-150.5 °C V.p. £1.33 ´ 10-2 mPa (50 °C) KOW logP = 4.01 Solubility In water 0.18 ppm (25 °C). Stability DT50 30-60 d (50 °C).
COMMERCIALISATION
History Developed since 1992, by Rhône-Poulenc Agrochimie (now Bayer CropScience). Reported by K. Jikihara et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1997, 1, 73). Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Novel growth inhibitor, affecting cell elongation; target site not identified (S. K. Miller et al., Proc. Br. Crop Prot. Conf. - Weeds, 2001, 2, 569). Mode of action Symptoms (in Echinochloa spp.) consist of chlorosis, reddish colouration of leaves and shoots, necrosis and death. Uses Control of Echinochloa spp., sedges and some broad-leaved weeds, in paddy rice, at rates down to 30 g/ha when applied pre-emergence. Formulation types WG; GR; SC; WP. Selected products: mixtures: 'Patful' (+ pyriminobac-methyl+ bensulfuron-methyl) (Kumiai)
OTHER PRODUCTS
'Samourai' (Bayer CropScience, Nihon Nohyaku); 'Thoroughbred' (Bayer CropScience, Sumitomo Chemical Takeda); 'Tredy' (Bayer CropScience, Nissan) mixtures: 'Homerun' (+ bensulfuron-methyl) (Hokko)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats and mice >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats and mice >2000 mg/kg. Minimal eye irritation, not a skin irritant (rabbits); not a skin sensitiser (guinea pigs). Other Negative in Ames test. Not teratogenic.
ECOTOXICOLOGY
Fish LC50 (48 h) for carp >5 ppm.
|