|
nitenpyram
Insecticide
IRAC 4A; neonicotinoid
NOMENCLATURE
Common name nitenpyram (BSI, pa E-ISO)
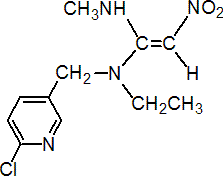
IUPAC name (E)-N-(6-chloro-3-pyridylmethyl)-N-ethyl-N'-methyl-2-nitrovinylidenediamine
Chemical Abstracts name N-[(6-chloro-3-pyridinyl)methyl]-N-ethyl-N'-methyl-2-nitro-1,1-ethenediamine
CAS RN [120738-89-8]; [150824-47-8] (E)- isomer Development codes TI-304 (Takeda); CGA 246916 (Ciba-Geigy)
PHYSICAL CHEMISTRY
Mol. wt. 270.7 M.f. C11H15ClN4O2 Form Pale yellow crystals. M.p. 82.0 °C V.p. 1.1 ´ 10-6 mPa (20 °C) KOW logP = -0.66 (25 °C, unstated pH) Henry 3.54 ´ 10-13 Pa m3 mol-1 (calc.) S.g./density 1.40 (26 °C) Solubility In water >590 g/l (pH 7.0, 20 °C). In dichloromethane and methanol >1000, chloroform 700, acetone 290, ethyl acetate 34.7, toluene 10.6, xylene 4.5, hexane 0.00470 (all in g/l, 20 °C). Stability Stable at 150 °C. Stable to hydrolysis at pH 3, 5 and 7; DT50 696 (pH 9, 25 °C). pKa pKa1 3.1, pKa2 11.5
COMMERCIALISATION
History Reported by Minamida et al. (J. Pestic. Sci., 18, 41 (1993)). Under evaluation for crop protection use since 1989; introduced by Takeda Chemical Industries Ltd (now Sumitomo Chemical Takeda Agro Company Ltd), and first launched in 1995. Also developed jointly with Novartis Animal Health Inc., for flea control. Manufacturers Sumitomo Chemical Takeda
APPLICATIONS
Biochemistry Agonist of the nicotinic acetylcholine receptor, affecting cholinergic transmissions in the insect central nervous system. Mode of action Systemic insecticide with translaminar activity and with contact and stomach action. Uses Control of aphids, thrips, leafhoppers, whitefly, and other sucking insects on rice and glasshouse crops. On rice, applied at 15-75 g/ha (foliar), 75-100 g/ha (dust) or 300-400 g/ha (soil treatment). Also for control of fleas on cats and dogs. Formulation types DP; GR; SP. Selected products: 'Bestguard' (Sumitomo Chemical Takeda)
OTHER PRODUCTS
'Capstar' (flea control) (Sumitomo Chemical Takeda, Novartis A H); 'Programa' (flea control, Japan) (Sumitomo Chemical Takeda, Novartis A H); 'Takestar' (flea control, Japan) (Sumitomo Chemical Takeda, Novartis A H)
ANALYSIS
Product and residue analysis by hplc. Details from Takeda.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 1680, female rats 1575, male mice 867, female mice 1281 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Very slightly irritating to eyes; not irritating to skin (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >5.8 g/m3 air. NOEL (2 y) for male rats 129, female rats 53.7 mg/kg b.w. daily; (1 y) for male and female dogs 60 mg/kg b.w. daily. Other Not oncogenic (rats, mice). Not teratogenic (rats, rabbits). No effect on reproductive performance (rats). Non-mutagenic (4 tests).
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2250, mallard ducks 1124 mg/kg. Dietary LC50 (5 d) for bobwhite quail and mallard ducks >5620 ppm. Fish LC50 (96 h) for carp >1000 mg/l. Daphnia LC50 (24 h) >10 000 mg/l. Algae EbC50 (72 h) for Selenastrum capricornutum 26 mg/l. NOEC (120 h) 6.25 mg/l. Worms LC50 (14 d) 32.2 mg/kg.
ENVIRONMENTAL FATE
Soil/Environment DT50 in soil 1-15 d, depending on soil type.
|