|
metosulam
Herbicide
HRAC B WSSA 2; triazolopyrimidine
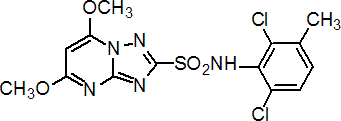
NOMENCLATURE
Common name metosulam (BSI, pa E-ISO, ANSI); métosulame ((m) pa F-ISO)
IUPAC name 2',6'-dichloro-5,7-dimethoxy-3'-methyl[1,2,4]triazolo[1,5-a]pyrimidine-2-sulfonanilide
Chemical Abstracts name N-(2,6-dichloro-3-methylphenyl)-5,7-dimethoxy[1,2,4]triazolo[1,5-a]pyrimidine-2-sulfonamide
CAS RN [139528-85-1] Development codes XDE 511; DE 511; XRD 511 (all Dow)
PHYSICAL CHEMISTRY
Mol. wt. 418.3 M.f. C14H13Cl2N5O4S Form Cream to tan coloured powder. M.p. 210-211.5 ºC V.p. 4 ´ 10-10 mPa (20 ºC, Knudsen effusion) KOW logP = 0.9778 (distilled water), 2.12 (pH 5), 2.46 (pH 7), 3.08 (pH 9) (all at 20 ºC) S.g./density 1.49 (20 ºC) Solubility In water 200 (distilled water, pH 7.5), 100 (pH 5.0), 700 (pH 7.0), 5600 (pH 9.0) (all in mg/l, 20 ºC). In acetone, acetonitrile and dichloromethane >5.0 g/l; in n-octanol, hexane and toluene £0.2 g/l. Stability Under normal storage conditions, decomposes above m.p. Little photolytic instability (DT50 140 d - Xenon Arc); stable to hydrolysis across environmentally normal range. pKa 4.8
COMMERCIALISATION
History Herbicide reported by M. Snel et al., Meded. Fac. Landbouwwet. Rijksuniv. Gent, 1993, 58/32, 845. 'Sansac' (mixture with 2,4-D EHE) launched in Turkey in 1994. Manufacturers Dow AgroSciences
APPLICATIONS
Biochemistry Branched chain amino acid (leucine, isoleucine and valine) synthesis (ALS or AHAS) inhibitor. Selectivity in wheat is due to rapid metabolic deactivation. Mode of action Herbicide readily taken up by roots and foliage. Uses Post-emergence control of many important broad-leaved weeds (including Galium aparine, Stellaria media, and all members of the Brassicaceae) in wheat, barley and rye; and pre- or post-emergence control of many important broad-leaved weeds in maize (including Chenopodium spp., Amaranthus retroflexus, Solanum nigrum and Polygonum persicaria). Phytotoxicity Non-phytotoxic to crops for which use is recommended. Formulation types SC; SE; WG. Selected products: 'Sansac' (Turkey) (Dow AgroSciences, Bayer CropScience); 'Sinal' (Middle East/Africa) (Dow AgroSciences, Bayer CropScience)
OTHER PRODUCTS
'Eclipse' (Dow AgroSciences, Bayer CropScience); 'Tacco' (Dow AgroSciences, Bayer CropScience) mixtures: 'Barko' (+ atrazine) (Dow AgroSciences); 'Metto' (+ atrazine) (Dow AgroSciences, Bayer CropScience); 'Presto' (+ fluoroglycofen-ethyl) (Dow AgroSciences, Bayer CropScience); 'Sound' (+ 2,4-D) (Dow AgroSciences, Bayer CropScience); 'Diplôme' (+ flufenacet) (Bayer CropScience); 'Terano' (+ flufenacet) (Bayer CropScience) Discontinued products mixtures: 'EF1166' * (+ fluroxypyr) (Dow AgroSciences, Bayer)
ANALYSIS
An hplc procedure based on extractive methylation is available on request from Dow AgroSciences.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats and mice >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >1.9 mg/l. NOEL (2 y) for rats 5 mg/kg b.w. daily; (18 mo) for mice 1000 mg/kg b.w. daily. ADI 0.02 mg/kg b.w. Toxicity class WHO (a.i.) U
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks and bobwhite quail >2000 mg/kg. Fish LC50 (96 h) for rainbow trout, bluegill sunfish and fathead minnows >solubility limit of a.i. Daphnia LC50 (48 h) >solubility limit of a.i. Algae LC50 (72 h) for green algae 75 mg/l. Bees Not toxic to bees. LD50 (48 h) (oral) >50 mg/bee; (contact) >100 mg/bee. Worms LC50 (14 d) for earthworms >1000 ppm.
ENVIRONMENTAL FATE
Animals Metosulam is rapidly absorbed following oral administration (DT50 <1 h), extensively metabolised in rodents, much less in dogs, and excreted with metabolites 3-hydroxy (aliphatic oxidation) and 5-hydroxy (O-demethylation) in urine (DT50 54-60 h in rodents, 73 h in dogs). In vitro percutaneous absorption is very low in humans and rats (<1% of applied in 24 h). Plants Metosulam is poorly absorbed from foliar application to wheat (<5% of applied), so there is little residue accumulation. Metabolised by hydroxylation of the ring methyl, to give a 3-hydroxymethyl- metabolite and its glycoside; these are the only major products found besides the parent molecule. Soil/Environment Laboratory aerobic degradation DT50 averaged 6 d (4 soils) at 20 ºC and 40% moisture-holding capacity. Field DT50 in the 0-10 cm horizon has a mean value of 25 d. Metosulam degrades via the 5- and 7- hydroxy analogues to 5-amino-N-(2,6-dichloro-3-methylphenyl)-1H-1,2,4-triazole-3-sulfonamide and CO2. Mean Koc (9 soils) <500. Spring-treated lysimeters recorded no component >0.1 mg/l in percolate following two successive annual treatments at c. 25 g a.i./ha.
|