|
metconazole
Fungicide
FRAC 3, G1; DMI: triazole
NOMENCLATURE
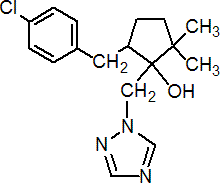
Common name metconazole (BSI, pa ISO (for cis:trans mixture))
IUPAC name (1RS,5RS;1RS,5SR)-5-(4-chlorobenzyl)-2,2-dimethyl-1-(1H-1,2,4-triazol-1-ylmethyl)cyclopentanol
Chemical Abstracts name 5-[(4-chlorophenyl)methyl]-2,2-dimethyl-1-(1H-1,2,4-triazol-1-ylmethyl)cyclopentanol
CAS RN [125116-23-6] unstated stereochemistry Development codes KNF-S-474 (Kureha); WL 148271 (Shell); AC 900,768 (Cyanamid); BAS 555 F (BASF)
PHYSICAL CHEMISTRY
Composition The common name metconazole applies to the (1RS,5RS;1RS,5SR)- isomers. Tech. material is a mixture of cis- and trans- isomers, predominantly cis-, (1RS,5SR) (hydroxy and benzyl groups on the same side of the cyclopentyl ring); both isomers are fungicidally active. Mol. wt. 319.8 M.f. C17H22ClN3O Form Off-white, odourless powder. M.p. 100.0-108.4 °C V.p. 2.1 ´ 10-5 mPa (20 ºC) KOW logP = 3.85 (20 ºC) Henry 2.21 ´ 10-7 Pa m3 mol-1 S.g./density 1.14 Solubility In water 30.4 mg/l (20 ºC). In methanol 403, acetone 363 (both mg/ml, 20 ºC). Stability Good thermal and hydrolytic stability.
COMMERCIALISATION
History Discovered by Kureha Chemical Industry Co., Ltd in 1986. Reported by A. J. Sampson et al. (Proc. Br. Crop Prot. Conf. - Pests Dis., 1992, 1, 419). Developed jointly with Shell Group (later American Cyanamid Co., then BASF AG); introduced by Cyanamid Agro in France in 1994. Patents EP 267778 Manufacturers Kureha
APPLICATIONS
Biochemistry Steroid demethylation (ergosterol biosynthesis) inhibitor. Both isomers are active. Mode of action Applied post-emergence, exhibits penetrant, local and acropetal systemicity. Uses Control of a wide range of foliar diseases on cereals, at 90 g/ha, and on other crops. It is particularly effective against Fusarium, Septoria and rust diseases on cereals. Phytotoxicity Exhibits some plant growth regulant activity such as thickening of leaves, stunting and darkening of leaf colour. Formulation types SL. Selected products: 'Caramba' (BASF)
OTHER PRODUCTS
'Cinch Pro' (BASF); 'Cinch' (BASF); 'Juventus 90' (BASF); 'Sirocco' (BASF); 'Sunorg Pro' (BASF); 'Sunorg' (BASF)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 660 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Not irritant to skin; slight irritant to eyes (rabbits). Not a skin sensitiser. Inhalation LC50 (4 h) for rats >5.6 mg/l. NOEL (104 w) for rats 4.8 mg/kg b.w. daily; (52 w) for dogs 11.1 mg/kg b.w. daily; (90 d) for mice 5.5, rats 6.8, dogs 2.5 mg/kg b.w. daily. ADI 0.048 mg/kg b.w. Other Negative in the Ames test. Toxicity class WHO (a.i.) III
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 787 mg/kg. Acute dietary LC50 for mallard ducks >5200 mg/kg. Fish LC50 (96 h) for rainbow trout 2.2, fathead minnow 3.9, common carp 3.99 mg/l. Daphnia LC50 (48 h) 4.2 mg/l. Algae EC50 (72 h) for Selenastrum capricornutum 1.7 mg/l. Bees Practically non-toxic to bees; oral LD50 (24 h) 90 mg/bee. Worms Practically non-toxic to earthworms.
ENVIRONMENTAL FATE
Soil/Environment Koc 726-1718 (five soil types). No pH dependence.
|