|
mepronil
Fungicide
FRAC 7, C2; carboxamide
NOMENCLATURE
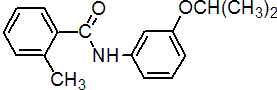
Common name mepronil (BSI, JMAF, draft E-ISO)
IUPAC name 3'-isopropoxy-o-toluanilide
Chemical Abstracts name 2-methyl-N-[3-(1-methylethoxy)phenyl]benzamide
CAS RN [55814-41-0] Development codes B1-2459; KCO-1 (Kumiai)
PHYSICAL CHEMISTRY
Composition Tech. is >94%. Mol. wt. 269.3 M.f. C17H19NO2 Form Colourless crystals. M.p. 92-93 °C V.p. 0.056 mPa (20 ºC) KOW logP = 3.66 Henry 1.19 ´ 10-3 Pa m3 mol-1 (calc.) Solubility In water 12.7 mg/l (20 ºC). In acetone >500, methanol >500, acetonitrile 314, benzene 282, hexane 1.1 (all in g/l, 20 ºC). Stability Stable to light, air, and heat. Stable in neutral, acidic, and weakly alkaline conditions, but hydrolysed in highly alkaline conditions. F.p. 225 °C
COMMERCIALISATION
History Reported by Kumiai Chemical Industry Co., Ltd. (I. Chiyomaru et al., ACS/CSJ Chem. Congr., Div. Pestic. Chem., Hawaii, No. 79 (1979); S. Kawada et al., Ann. Phytopath. Soc. Jap.,45(4), 547 (1979)). Reviewed by S. Doi (Jpn. Pestic. Inf., 1981, No. 38, p. 17) and introduced by Kumiai in 1981. Patents US 3937840; GB 1421112; JP 906789 Manufacturers Ihara/Kumiai; Sannong
APPLICATIONS
Biochemistry Inhibits succinic acid oxidation during metabolic respiration. Mode of action Systemic fungicide with protective and curative action. Uses Control of diseases caused by Basidiomycetes (e.g. Rhizoctonia, Puccinia, Typhula spp., etc.) in rice (at 0.5-0.75 kg/l), cereals (at 0.5-1.0 kg/l), potatoes, vegetables, cucumbers, sugar beet, fruit, vines, tobacco, turf grass (at 0.75-1.5 kg/l), ornamentals, etc. Also, when used as a seed or soil treatment, control of damping-off diseases of vegetables and tobacco. Formulation types DP; SC; WP. Selected products: 'Basitac' (Kumiai)
ANALYSIS
Product and residue analysis by glc; details available from Kumiai.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats and mice >10 000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits and rats >10 000 mg/kg. Non-irritating to skin and eyes (rabbits). Inhalation LC50 (6 h) for rats >1.32 mg/l. NOEL (2 y) for male rats 5.9, female rats 72.9, male mice 13.7, female mice 17.8 mg/kg b.w. daily. ADI 0.05 mg/kg. Other Non-mutagenic, non-teratogenic in rats and rabbits. Toxicity class WHO (a.i.) U; EPA (formulation) IV
ECOTOXICOLOGY
Birds Acute oral LD50 for hens >8000 mg/kg. Fish LC50 (96 h) for carp 8, rainbow trout 10 mg/l. Daphnia LC50 for D. carinata >10 mg/l. Bees Acute LD50 (oral) >0.1 mg/bee; (contact) >1 mg/bee.
ENVIRONMENTAL FATE
Animals Almost all of the dose applied to rats was excreted in urine and faeces within 96 h. Plants Almost all 14C applied to rice leaf sheath or leaf remained in the part treated, with some translocation upwards. Five metabolites were identified. Soil/Environment DT50 in flooded soil 46 d (volcanic sandy loam, pH 6.8, T.C. 1.47%), 50.5 d (alluvial, pH 6.5, T.C. 1.18%).
|