|
maneb
Fungicide
FRAC M3; multi-site: alkylenebis(dithiocarbamate)
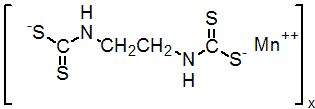
NOMENCLATURE
Common name maneb (BSI, E-ISO, JMAF); manèbe ((m) F-ISO)
IUPAC name manganese ethylenebis(dithiocarbamate) (polymeric)
Chemical Abstracts name [1,2-ethanediylbis[carbamodithioato](2-)]manganese
Other names MEB CAS RN [12427-38-2] EEC no. 235-654-8 Official codes ENT 14 875
PHYSICAL CHEMISTRY
Mol. wt. 265.3 M.f. C4H6MnN2S4 Form Yellow, crystalline solid. M.p. Decomposes, without melting, at 192-204 ºC V.p. Negligible at 20 ºC S.g./density 1.92. Solubility Practically insoluble in water and in common organic solvents. Soluble in chelating agents (e.g. sodium salts of ethylenediaminetetraacetic acid), with the formation of complexes. Stability Stable to light. Decomposes on prolonged exposure to air or moisture. On hydrolysis, DT50 <24 h (pH 5, 7 or 9). Etem is one of the products formed on contact with moisture (R. A. Ludwig et al., Can. J. Bot., 1955, 33, 42; C. W. Pluijgers et al., Tetrahedron Lett., 1971, p. 1371).
COMMERCIALISATION
History Introduced by E. I. du Pont de Nemours and Co. (who no longer manufacture or market it) and by Rohm & Haas Co. (now Dow AgroSciences). Patents US 2504404; US 2710822 both to Du Pont Manufacturers Cerexagri; Crystal; Drexel
APPLICATIONS
Biochemistry Non-specific thiol reactant, inhibiting respiration. Mode of action Fungicide with protective action. Uses Control of many fungal diseases (e.g. blight, leaf spot, rust, downy mildew, scab, etc.) in field crops, fruit, nuts, vegetables, ornamentals, turf, etc. Particular uses include control of early and late blights of potatoes and tomatoes; Rhizoctonia solani and Streptomyces scabies on seed potatoes; leaf spot diseases on celery, beet and currants; rusts on cereals, roses, carnations, asparagus, beans, apples, plums and currants; downy mildews on hops, vines, onions, ornamentals and tobacco; Gloeodes pomigena, Glomerella cingulata, Microthyriella rubi and Physalospora obtusa on apples; scab on apples and pears; sigatoka disease (Cercospora musae) on bananas; anthracnose of beans; tulip fire; needle cast in forestry; and many seed-borne diseases of cereals. Used for foliar application or as a seed treatment. Phytotoxicity Morello cherries and some varieties of apple and cucurbits may be injured. Formulation types SC; WG; WP; Seed treatment. Selected products: 'Manex' (Crystal, Griffin); 'Policritt' (Siapa); mixtures: 'Brestan' (+ fentin acetate) (Bayer CropScience)
OTHER PRODUCTS
'Dithane M-22' (Dow AgroSciences); 'Manzate' (DuPont); 'Agroneb' (AgroSan); 'Granéor' (Cerexagri); 'Manzi' (Drexel); 'Trimanzone' (Intracrop); 'X-Spor' (United Phosphorus) mixtures: 'Bordeaux M' (+ Bordeaux mixture) (Nufarm SA, Nufarm Americas); 'Dustret' (+ streptomycin sesquisulfate) (Agsco); 'Enhance' (+ carboxin+ gamma-HCH) (Gustafson); 'Granol Plus' (+ thiabendazole+ gamma-HCH) (Wilbur-Ellis); 'Granox' (+ thiabendazole) (Agriliance); 'MC Flowable' (+ carbendazim) (United Phosphorus); 'Pro-Tex' (+ fentin hydroxide) (Griffin); 'Seed Treatment For Potatoes' (+ streptomycin sesquisulfate) (Helena); 'Sirdate P' (+ oxadixyl+ cymoxanil) (Griffin) Discontinued products: 'Manex' * (with zinc) (Chiltern); 'Mazin' * (Unicrop); 'Polyram M' * (BASF); 'Spirit' * (Headland) mixtures: 'Ashlade Mancarb Plus' * (+ carbendazim+ chlorothalonil) (Ashlade); 'Ashlade Mancarb' * (+ carbendazim) (Nufarm Whyte); 'Ashlade SMC' * (+ sulfur+ copper oxychloride) (Ashlade); 'Bolda' * (+ sulfur+ carbendazim) (Atlas); 'Bordeaux MZ' * (+ zineb+ Bordeaux mixture) (Nufarm Americas); 'Cosmic' * (+ tridemorph+ carbendazim) (BASF); 'Dual' * (+ carbendazim) (Headland); 'Legion' * (+ carbendazim) (Tripart); 'Multi-W' * (+ carbendazim) (PBI); 'New Squadron' * (+ carbendazim) (Quadrangle); 'Senator' * (+ sulfur+ copper oxychloride) (Tripart); 'Trimanzone WP' * (+ zineb+ ferbam) (Intracrop); 'Victor' * (+ carbendazim+ chlorothalonil) (Tripart)
ANALYSIS
Product analysis by decomposition with acid and measurement of the liberated carbon disulfide, either by glc or by colorimetry of a derivative (AOAC Methods, 17th Ed., 965.15, 991.33; CIPAC Handbook, 1992, E, 116-122); in mixture with copper compounds, or fentin salts, by similar methods or by rplc with u.v. detection (ibid., 1998, H, 100; 1992, E, 89; 1988, D, 95); determination of manganese, ibid., 1994, F, 232; ETU content by hplc with u.v. detection, or by paper chromatography (ibid., 1994, F, 399). Residues determined by reaction to form carbon disulfide which is measured by standard methods (Analyst (London), 1981, 106, 782; Pestic. Anal. Man., 1979, II; Manu. Pestic. Residue Anal., 1987, I, S21; Anal. Methods Residues Pestic., 1988, Part II).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 68, 70 (see part 2 of the Bibliography). class 3 Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats and rabbits >5000 mg/kg. Moderate eye irritant; non-irritating to skin (rabbits). May cause irritation to nose and throat. Inhalation LC50 (4 h) for rats >3.8 mg/l air. NOEL In 2 y feeding trials, no ill-effect observed in rats receiving 250 mg/kg diet, and at 2500 mg/kg diet showed signs of toxicity; in 1 y trials with dogs, no effect observed at 20 mg/kg daily but toxicity observed at 75 mg/kg daily. ADI (JMPR) 0.03 mg/kg b.w. (group ADI with mancozeb, metiram and zineb); ethylenethiourea 0.004 mg/kg b.w. [1993]. Other At very high levels, maneb has caused birth defects in test animals; ethylenethiourea, a trace contaminant and degradation product of maneb, has caused thyroid effects, tumours and birth defects in laboratory animals. Toxicity class WHO (a.i.) U; EPA (formulation) IV EC classification Xi; R37| R43
ECOTOXICOLOGY
Birds Dietary LC50 (8 d) for mallard ducks and bobwhite quail >10 000 mg/kg diet. Fish LC50 (48 h) for carp 1.8 mg/l. Bees Not toxic to bees.
ENVIRONMENTAL FATE
EHC 78 (WHO, 1988; general review of dithiocarbamates). Animals In rats, metabolites included ethylenediamine, ethylenebis(thiuram) monosulfide and ethylenebis(thiourea) (H. Seidler et al., Nahrung, 1971, 15, 177-185). Plants In plants, the principal metabolite is ethylenethiourea, which rapidly undergoes further metabolism. Ethylenethiuram monosulfide, ethylenethiuram disulfide, and sulfur are also metabolites. Soil/Environment Rapidly degraded in the environment by hydrolysis, oxidation, photolysis, and metabolism. Soil DT50 c. 25 d (loamy sand in dark, aerobic conditions).
|