|
Magnesium Phosphide
Insecticide, rodenticide
IRAC 8B; fumigant
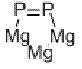
NOMENCLATURE
magnesium phosphide
IUPAC name magnesium phosphide
Chemical Abstracts name magnesium phosphide
CAS RN [12057-74-8] EEC no. 235-023-7
PHYSICAL CHEMISTRY
magnesium phosphide
Mol. wt. 134.9 M.f. Mg3P2 Form Yellow-green crystals. M.p. >750 °C S.g./density 2.055 Stability Stable when dry, but reacts with atmospheric moisture, and violently with acids, producing phosphine; used to generate this fumigant, reacting more rapidly than aluminium phosphide.
COMMERCIALISATION
magnesium phosphide
History Introduced by Degesch AG to generate phosphine and so fumigate stored foodstuffs. Patents DE 923999 to Edmund Manufacturers Detia Freyberg; United Phosphorus
APPLICATIONS
magnesium phosphide
Mode of action Insecticide and rodenticide which is a respiratory, metabolic, and nerve poison. Liberates phosphine, which is the toxicant. Uses As for aluminium phosphide. Also used for control of moles, voles, rats, hamsters, and rabbits by fumigation of burrows. Phytotoxicity Living plants, fresh vegetables and fruits, with few exceptions, should not be fumigated. Formulation types GE; Fumigant. Selected products: 'Magnaphos' (United Phosphorus); 'Magtoxin' (Detia Degesch)
OTHER PRODUCTS
magnesium phosphide
'Degesch Plates' (Detia Degesch); 'Fumi-Cel' (Degesch America); 'Fumi-Strip' (Degesch America) Discontinued products: 'Detiaphos' * (Detia Degesch)
ANALYSIS
magnesium phosphide
Product analysis by determining the phosphine liberated on treatment with acid, by glc (T. Dumas, J. Assoc. Off. Anal. Chem., 1978, 61, 51) or phosphate produced after reaction with bromine water (R. B. Bruce et al., J. Agric. Food Chem., 1962, 10, 18).
MAMMALIAN TOXICOLOGY
magnesium phosphide
Oral Acute oral LD50 for rats 11.2 mg/kg. Toxicity class WHO (a.i.) FM EC classification F; R15/29| T+; R28| N; R50
ENVIRONMENTAL FATE
EHC 73 (WHO, 1988; a review of phosphine and metal phosphides). Animals In mammals, phosphine is probably metabolised to non-toxic phosphates. Plants In stored products, phosphine undergoes oxidation to phosphoric acid.
|