|
lenacil
Herbicide
HRAC C1 WSSA 5; uracil
NOMENCLATURE
Common name lenacil (BSI, E-ISO, ANSI, WSSA, JMAF); lenacile ((m) F-ISO)
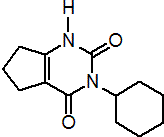
IUPAC name 3-cyclohexyl-1,5,6,7-tetrahydrocyclopentapyrimidine-2,4(3H)-dione
Chemical Abstracts name 3-cyclohexyl-6,7-dihydro-1H-cyclopentapyrimidine-2,4(3H,5H)-dione
Other names 3-cyclohexyl-5,6-trimethyleneuracil CAS RN [2164-08-1] Development codes DPX-B634
PHYSICAL CHEMISTRY
Mol. wt. 234.3 M.f. C13H18N2O2 Form White crystalline solid. M.p. 315.6-316.8 ºC V.p. 2 ´ 10-4 mPa (25 ºC) KOW logP = 2.31 Henry 2.11 ´ 10-5 Pa m3 mol-1 (calc.) S.g./density 1.32 Solubility In distilled water 3 ppm (25 ºC). Slightly soluble in most organic solvents, e.g. toluene 80, n- hexane 1.3, acetone 690, methanol 1500, ethyl acetate 690, dichloromethane 2000 (all in ppm, 20 ºC). Stability Stable up to the melting point. Stable in water and in aqueous acids. Decomposed by hot alkalis. pKa 10.3, v. weak acid
COMMERCIALISATION
History Herbicide reported by G. W. Cussans (Proc. Br. Weed Control Conf., 7th, 1964, 2, 671). Introduced by E. I. du Pont de Nemours Co. Patents US 3235360 Manufacturers DuPont; Richter Gedeon
APPLICATIONS
Biochemistry Photosynthetic electron transport inhibitor at the photosystem II receptor site. Mode of action Selective, systemic herbicide, absorbed by the roots. Uses Control of annual grass and broad-leaved weeds in sugar beet, fodder beet, beetroot, sweet potatoes, spinach, strawberries, flax, black salsify, and ornamental plants and shrubs. Applied either pre-plant soil-incorporated or pre-emergence. at 0.12-2.7 kg/ha. Formulation types SC; WP. Selected products: 'Venzar' (DuPont)
OTHER PRODUCTS
'Lenamon' (DuPont); 'Verape' (DuPont) mixtures: 'Advizor S' (+ chloridazon) (DuPont); 'Seppic lin' (+ linuron) (DuPont); 'Agricola Lens' (+ phenmedipham) (Agricola); 'Lenapac' (+ chloridazon) (Hokko, Maruwa) Discontinued products: 'Adol' * (Richter Gedeon) mixtures: 'DUK 880' * (+ phenmedipham) (DuPont); 'Merpelan AZ' * (+ isocarbamid) (Bayer); 'Stefes 880' * (+ phenmedipham) (Stefes); 'Terratop' * (+ isocarbamid) (Bayer)
ANALYSIS
Product determined by glc. Residues determined by glc (H. L. Pease, J. Sci. Food Agric.,1966, 17, 121; H. J. Jarczyk, Pflanzenschutz-Nachr. (Eng. Ed.), 1977, 30, 3) or by hplc (T. H. Byast, J. Chromatogr.,1977, 134, 211).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >11 000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >5000 mg/kg; mild eye irritant (rabbits, WP formulation). Not a skin sensitiser. Inhalation LC50 (1 h) for rats >5.2 mg/l. NOEL In 2 y feeding trials with rats, no adverse effect observed. Other Not considered carcinogenic, embryotoxic or teratogenic. Toxicity class WHO (a.i.) U; EPA (formulation) IV
ECOTOXICOLOGY
Birds Dietary LC50 (8 d) for bobwhite quail 2300, Pekin ducks >5620 ppm. Fish LC50 (96 h) for bluegill sunfish 100-1000, minnow >2.0, trout 135 mg/l. LC50 (21 d) for trout >2.3 mg/l. Daphnia LC50 (48 h) >8.4 mg/l. Algae NOEL (120 h) for Selenastrum capricornutum 0.010 mg/l. Bees Not toxic to bees; LD50 (contact) >25 mg/bee; LC50 (oral) >1000 ppm. Worms LC50 for earthworms >10 000 mg/kg.
ENVIRONMENTAL FATE
Plants In sugar beet, hydroxylation followed by glucose conjugation. Soil/Environment Degradation in soil involves oxidation of lenacil in the cyclopentene ring. Soil DT50 3 months. Kd 0.63-4.6; Koc 136-417
|