|
lambda-cyhalothrin
Insecticide
IRAC 3; pyrethroid
NOMENCLATURE
Common name lambda-cyhalothrin (BSI, draft E-ISO); lambda-cyhalothrine ((f) draft F-ISO)
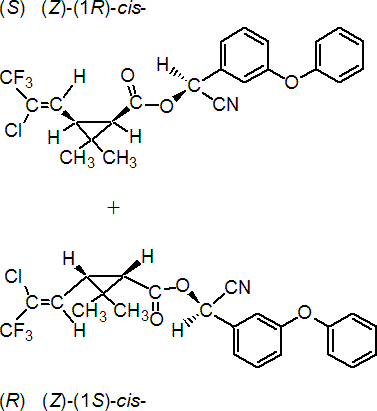
IUPAC name A reaction product comprising equal quantities of (S)-a-cyano-3-phenoxybenzyl (Z)-(1R,3R)-3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropanecarboxylate and (R)-a-cyano-3-phenoxybenzyl (Z)-(1S,3S)-3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropanecarboxylate
Roth: (S)-a-cyano-3-phenoxybenzyl (Z)-(1R)-cis-3-(2-chloro-3,3,3-trifluoropropenyl)-2,2-dimethylcyclopropanecarboxylate and (R)-a-cyano-3-phenoxybenzyl (Z)-(1S)-cis-3-(2-chloro-3,3,3-trifluoropropenyl)-2,2-dimethylcyclopropanecarboxylate (1:1)
Chemical Abstracts name [1a(S*),3a(Z)]-(?-cyano(3-phenoxyphenyl)methyl 3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylate
CAS RN [91465-08-6] EEC no. ELINCS: 415-130-7 Development codes PP321; ICIA0321 (both ICI) Official codes OMS 3021
PHYSICAL CHEMISTRY
Composition Tech. grade is c. 81% pure. Mol. wt. 449.9 M.f. C23H19ClF3NO3 Form Colourless solid; (tech. is a dark brown/green solidified melt). M.p. 49.2 ºC; (tech., 47.5-48.5 ºC) B.p. Does not boil at atmospheric pressure V.p. 2 ´ 10-4 mPa (20 ºC, est.); 2 ´ 10-1 mPa (60 ºC, interpolated) KOW logP = 7 (20 ºC) Henry 2 ´ 10-2 Pa m3 mol-1 S.g./density 1.33 g/ml (25 ºC) Solubility In water 0.005 mg/l (20 °C). In acetone, methanol, toluene, hexane, ethyl acetate >500 g/l. Stability Stable to light. Stable on storage for more than 6 months at 15-25 ºC. pKa >9 (hydrolysis prevents measurement) F.p. 185 °C (tech., Pensky-Martens closed cup)
COMMERCIALISATION
History Insecticide reported by A. R. Jutsum et al. (Proc. Br. Crop Prot. Conf. - Pests Dis., 1984, 2, 421). Introduced in Central America and Far East (1985) by ICI Agrochemicals (now Syngenta AG). Patents EP 107296; EP 106469 Manufacturers Agrochem; Hesenta; Jiangsu Yangnong; Sharda; Syngenta; Tide
APPLICATIONS
Biochemistry Acts on the nervous system of insects, disturbs the function of neurons by interaction with the sodium channel. Mode of action Non-systemic insecticide with contact and stomach action, and repellent properties. Gives rapid knockdown and long residual activity. Uses Control of a wide spectrum of insect pests, e.g. aphids, Colorado beetles, thrips, Lepidoptera larvae, Coleoptera larvae and adults, etc., in cereals, hops, ornamentals, potatoes, vegetables, cotton, and other crops. Provides good control of insect-borne plant viruses, at 2-5 g/ha. Also used for control of insect pests in public health. Formulation types CS; EC; EW; UL; WG; WP. Selected products: 'Icon' (Syngenta); 'Karate' (Syngenta); 'Warrior' (Syngenta); 'Phoenix' (Vapco); 'SFK' (Trithin); mixtures: 'Okapi' (+ pirimicarb) (Syngenta)
OTHER PRODUCTS
'Demand' (Syngenta, Sorex); 'Hallmark' (Syngenta); 'Kung Fu' (Syngenta); 'Matador' (Syngenta); 'Scimitar' (Syngenta); 'Katron' (Agrochem); 'Tornado' (public health) (Vapco) mixtures: 'Dovetail' (+ pirimicarb) (Syngenta); 'Karate K' (+ pirimicarb) (Syngenta); 'Tornado-Forte+' (+ piperonyl butoxide+ tetramethrin) (public health) (Vapco) Discontinued products: 'Hero' * (Zeneca) mixtures: 'Karapp' * (+ buprofezin) (Zeneca)
ANALYSIS
Product analysis of pyrethroids reviewed by E. Papadopoulou-Mourkidou in Comp. Analyt. Profiles. By capillary glc with FID (CIPAC Handbook, 1992, E, 49-57). Residues determined by glc with ECD (AOAC Methods, 17th Ed., 998.01). Details available from Syngenta.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 79, female rats 56. Skin and eye Acute percutaneous LD50 for rats 632-696 mg/kg. Mild eye irritant; non-irritant to skin (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats 0.06 mg/l air (total particulate). NOEL (1 y) for dogs 0.5 mg/kg daily. ADI 0.005 mg/kg. Other Non-mutagenic in the Ames test. Toxicity class WHO (a.i.) II; EPA (formulation) II ('Karate', 'Warrior') EC classification T+; R26| T; R25| Xn; R21| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >3950 mg/kg. Dietary LC50 for quail >5300 mg/kg. No accumulation of residues in eggs or tissues. Fish LC50 (96 h) for bluegill sunfish 0.21, rainbow trout 0.36 mg/l. Daphnia EC50 (48 h) 0.36 mg/l. Algae ErC50 (96 h) for Selenastrum capricornutum >1000 mg/l. Other aquatic spp. Intrinsic toxicity to aquatic organisms is greatly reduced by rapid loss from the water by adsorption and degradation. Bees LD50 (oral) 38 ng/bee; (contact) 909 ng/bee. Worms LC50 for Eisenia foetida >1000 mg/kg soil. Other beneficial spp. Toxic to some non-target arthropods. Effects under field conditions reduced, with rapid recovery.
ENVIRONMENTAL FATE
Animals In rats, following oral administration, rapidly eliminated in urine and faeces. The ester group is hydrolysed, both moieties forming polar conjugates. Plants For details of metabolism of lambda-cyhalothrin in cotton and soya bean leaves, see D. A. French & J. P. Leahey, Proc. Br. Crop Prot. Conf. - Pests Dis., 1990, 3, 1029-1034. Soil/Environment Rapidly degraded in soil; DT50 for microbial degradation 23-82 d, for field soil 6-40 d. Strongly adsorbed to soil and sediment organic matter, Koc 330 000. Negligible potential for leaching of lambda-cyhalothrin and its degradation products through soil. Rapid dissipation from water in aquatic systems. DT50 for dissipation from surface waters in lab. water-sediment systems 5-11 h; in a microcosm DT50 <3 h. Rapid and extensive degradation of parent compound in aquatic systems; DT50 for degradation in lab. water-sediment systems 7-15 d; in a microcosm DT50 <3 h, DT90 <3 d.
|