|
kasugamycin
See also The BioPesticide Manual, 2nd Ed., entry 2:120
Fungicide, bactericide
FRAC 24, D3; hexopyranosyl antibiotic
NOMENCLATURE
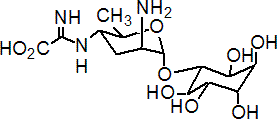
kasugamycin
Common name kasugamycin (JMAF)
CAS RN [6980-18-3]
kasugamycin hydrochloride hydrate
IUPAC name 1L-1,3,4/2,5,6-1-deoxy-2,3,4,5,6-pentahydroxycyclohexyl 2-amino-2,3,4,6-tetradeoxy-4-(a-iminoglycino)-a-D-arabino-hexopyranoside hydrochloride hydrate; [5-amino-2-methyl-6-(2,3,4,5,6-pentahydroxycyclohexyloxy)tetrahydropyran-3-yl]amino-a-iminoacetic acid hydrochloride hydrate
Chemical Abstracts name 3-O-[2-amino-4-[(carboxyiminomethyl)amino]-2,3,4,6-tetradeoxy-a-D-arabino-hexopyranosyl]-D-chiro-inositol hydrochloride hydrate
CAS RN [19408-46-9]
PHYSICAL CHEMISTRY
kasugamycin
Mol. wt. 379.4 M.f. C14H25N3O9 pKa pKa1 3.23, pKa2 7.73, pKa3 11.0
kasugamycin hydrochloride hydrate
Mol. wt. 433.8 M.f. C14H28ClN3O10 Form Colourless needle crystals. M.p. 202-204 ºC (decomp.) V.p. <1.3 ´ 10-2 mPa (25 ºC) KOW logP <1 Henry <2.9 ´ 10-8 Pa m3 mol-1 (calc.) S.g./density 0.43 g/cm3 (25 ºC) Solubility In water 228 g/l (pH 7, 25 ºC). In methanol 2.76, acetone, xylene <1 (all in mg/kg, 25 ºC). Stability Very stable at room temperature. Stable in weak acids, but unstable in strong acids and alkalis. DT50 (50 ºC) 47 d (pH 5), 14 d (pH 9). Specific rotation [a]D25 +120?(c 1.6 H2O)
COMMERCIALISATION
Production By fermentation of Streptomyces kasugaensis. History Kasugamycin was discovered by H. Umezawa et al. (J. Antibiot. (Tokyo), 1965, 18, 101), fungicidal activity reported by T. Ishiyama et al. (ibid., p. 115). Introduced by the Institute of Microbial Chemistry and by Hokko Chemical Industry Co., Ltd, and first marketed in 1965. Patents JP 42006818; BE 657659; GB 1094566 Manufacturers Hokko
APPLICATIONS
kasugamycin hydrochloride hydrate
Biochemistry Protein synthesis inhibitor. Inhibits binding of Met-RNA to the mRNA-30S complex, thereby preventing amino acid incorporation. Mode of action Systemic fungicide and bactericide with protective and curative action. Inhibits hyphal growth of Pyricularia oryzae on rice, preventing lesion development; comparatively weak inhibitory action to spore germination, appressoria formation on the plant surface or penetration into the epidermal cell. Rapidly taken up into plant tissue and translocated. In contrast, against Cladosporium fulvum on tomatoes, inhibition of sporulation is strong, but inhibition of hyphal growth is weak. Uses Control of fungal and bacterial diseases affecting rice, vegetables and fruit. On rice, it controls diseases caused by Pyricularia oryzae and Burkholderia glumae (bacterial grain rot), with ground and aerial applications, at 20-30 g/ha, and seedling diseases caused by various bacterial pathogens, at 0.3-0.6 g/box. In sugar beet, it controls Cercospora beticola, at 80-100 g/ha. Also used to control plant diseases in various crops: Pseudomonas syringae pv. lachrymans in cucumbers, at 30-60 g/ha; Colletotrichum lagenarium in melons and water melons; Cladosporium fulvum and Corynebacterium michiganense in tomatoes, at 20-40 g/ha; Mycovellosiella spp. in aubergines; Cercospora spp. in celery; Erwinia carotovora subsp. carotovora in potatoes, carrots and onions; Pseudomonas syringae pv. coronafaciens (halo blight) in kidney beans, at 40-60 g/ha; Xanthomonas spp. in paprika; Venturia sp. in apples; Pseudomonas marginalis pv. marginalis in kiwifruit; etc. Phytotoxicity Non-phytotoxic to rice, tomatoes, sugar beet, potatoes and other vegetables, but slight injury has been noted to peas, beans, soya beans, grapes, citrus, and apples. Formulation types DP; GR; SL; UL; WP. Compatibility Incompatible with pesticides which are strongly alkaline. Selected products: 'Kasugamin' (Hokko); 'Kasumin' (Hokko)
OTHER PRODUCTS
kasugamycin
Mixtures: 'Kasu-rab-valida-sumi' (+ phthalide+ validamycin+ fenitrothion) (Hokko)
kasugamycin hydrochloride hydrate
Mixtures: 'Kasai' (+ phthalide) (Hokko); 'Kasu-rabcide' (+ phthalide) (Hokko); 'Kasu-rab-sumibassa' (+ phthalide+ fenitrothion+ fenobucarb) (Hokko); 'Kasu-rab-validatrebon' (+ phthalide+ validamycin+ etofenprox) (Hokko); 'Kasu-ran' (+ copper oxychloride) (Hokko) Discontinued products mixtures: 'Kasumin Bordeaux' * (+ oxine-copper+ copper oxychloride) (Hokko)
ANALYSIS
Product analysis by cup assay with Pseudomonas fluorescens (NIHJ B-254). Residues determined by cup assay with Pyricularia oryzae (P2) (J. Antibiot. (Tokyo), 1968, 21, 49).
MAMMALIAN TOXICOLOGY
kasugamycin hydrochloride hydrate
Oral Acute oral LD50 for male rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Non-irritating to eyes and skin (rabbits). Inhalation LC50 (4 h) for rats >2.4 mg/l. NOEL (2 y) for rats 300, dogs 800 mg/kg diet. Other Non-mutagenic and non-teratogenic in rats, and without effect on reproduction. Toxicity class WHO (a.i.) U; EPA (formulation) IV
ECOTOXICOLOGY
kasugamycin hydrochloride hydrate
Birds Acute oral LD50 for male Japanese quail >4000 mg/kg. Fish LC50 (48 h) for carp and goldfish >40 mg/l. Daphnia LC50 (6 h) >40 mg/l. Bees LD50 (contact) >40 mg/bee.
ENVIRONMENTAL FATE
Animals Kasugamycin hydrochloride hydrate orally administered to rabbits was mostly excreted in the urine within 24 h. When injected intravenously to dogs, it was mostly excreted within 8 h. After oral administration to rats at 200 mg/kg, no residues were detected in eleven organs or blood; 96% of administered dose remained in the digestive tract 1 h after administration. Plants Degraded to kasugamycinic acid and kasuganobiosamine; finally degraded to ammonia, oxalic acid, CO2 and water. Soil/Environment Degradation proceeds as in plants.
|