|
ipconazole
Fungicide
FRAC 3, G1; DMI: triazole
NOMENCLATURE
Common name ipconazole (BSI, pa E-ISO)
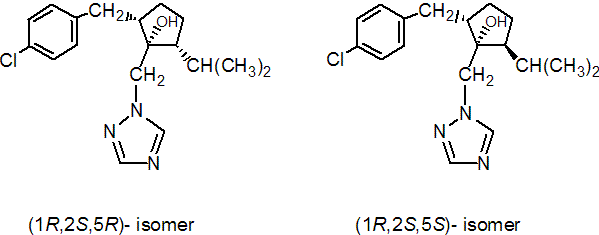
IUPAC name (1RS,2SR,5RS;1RS,2SR,5SR)-2-(4-chlorobenzyl)-5-isopropyl-1-(1H-1,2,4-triazol-1-ylmethyl)cyclopentanol
Chemical Abstracts name 2-[(4-chlorophenyl)methyl]-5-(1-methylethyl)-1-(1H-1,2,4-triazol-1-ylmethyl)cyclopentanol
CAS RN [125225-28-7] unstated stereochemistry Development codes KNF-317 (Kureha)
PHYSICAL CHEMISTRY
Composition The common name ipconazole refers to a mixture of the (1RS,2SR,5RS) isomer pair (I) (hydroxy, benzyl and isopropyl groups on the same side of the cyclopentyl ring) and the (1RS,2SR,5SR) isomer pair (II). Mol. wt. 333.9 M.f. C18H24ClN3O Form Colourless crystals. M.p. 88-90 ºC V.p. 3.58 ´ 10-3 mPa (25 ºC) (isomer pair I); 6.99 ´ 10-3 mPa (25 ºC) (isomer pair II) KOW logP = 4.21 (25 ºC) Solubility In water 6.93 mg/l (20 ºC). Stability Good thermal and hydrolytic stability.
COMMERCIALISATION
Patents EP 0267778; US 4938792 Manufacturers Kureha
APPLICATIONS
Biochemistry Inhibitor of ergosterol biosynthesis. Uses Control of a wide range of seed diseases in rice and other crops. It is particularly effective against Gibberella fujikuroi ('Bakanae' disease), Cochliobolus miyabeanus (Helminthosporium leaf spot) and Pyricularia oryzae (blast) on rice. The component isomer pairs are of equal activity. Formulation types EC; SC. Selected products: 'Techlead' (Kureha); mixtures: 'Befran-Seed' (+ iminoctadine triacetate) (Kureha, Dainippon); 'Techlead-C' (+ copper hydroxide) (Kureha, Kumiai)
OTHER PRODUCTS
'Vortex' (Gustafson)
ANALYSIS
Product analysis by hplc or glc.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 1338 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Not irritating to skin; slightly irritant to eyes (rabbits). Not a skin sensitiser.
ECOTOXICOLOGY
Fish LC50 (48 h) for carp 2.5 mg/l. Other aquatic spp. LC50 (3 h) for Moina macrocopa >40 mg/l.
ENVIRONMENTAL FATE
Animals Metabolites in the rat are 2-(4-chlorobenzyl)-5-(1-hydroxy-1-methylethyl)-1-(1H-1,2,4-triazol-1-ylmethyl)cyclopentanol, 2-(4-chlorobenzyl)-5-(2-hydroxy-1-methylethyl)-1-(1H-1,2,4-triazol-1-ylmethyl)cyclopentanol and 1,2,4-triazole. Plants In rice, metabolites are 2-(4-chlorobenzyl)-5-(1-hydroxy-1-methylethyl)-1-(1H-1,2,4-triazol-1-ylmethyl)cyclopentanol, 2-(4-chlorobenzyl)-5-(2-hydroxy-1-methylethyl)-1-(1H-1,2,4-triazol-1-ylmethyl)cyclopentanol and 2-[1-(4-chlorophenyl)hydroxymethyl]-5-isopropyl-1-(1H-1,2,4-triazol-1-ylmethyl)cyclopentanol.
|