|
inabenfide
Plant growth regulator
NOMENCLATURE
Common name inabenfide (BSI, draft E-ISO, (m) draft F-ISO)
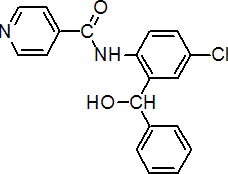
IUPAC name 4'-chloro-2'-(a-hydroxybenzyl)isonicotinanilide
Chemical Abstracts name N-[4-chloro-2-(hydroxyphenylmethyl)phenyl]-4-pyridinecarboxamide
CAS RN [82211-24-3] unstated stereochemistry Development codes CGR-811 (Chugai)
PHYSICAL CHEMISTRY
Mol. wt. 338.8 M.f. C19H15ClN2O2 Form Pale yellow-brown or colourless crystals. M.p. 210-212 ºC V.p. 0.063 mPa (20 ºC) KOW logP = 3.13 Solubility In water 1 mg/l (30 ºC). In acetone 3.6, ethyl acetate 1.43, xylene 0.58, methanol 2.35, ethanol 1.61, chloroform 0.59, dimethylformamide 6.72, acetonitrile 0.58, tetrahydrofuran 1.61, hexane 8 ´ 10-4 (all in g/l, 30 ºC). Stability Stable to heat and sunlight. Slightly unstable in alkaline media; after 2 weeks at 40 ºC, hydrolysed 16.2% (pH 2), 49.5% (pH 5), 83.9% (pH 7), 100% (pH 11).
COMMERCIALISATION
History Plant growth regulator reported by K. Nakamura (Jpn. Pestic. Inf., 1987, No. 51, p. 23). Introduced in Japan (1986) by Chugai Pharmaceutical Co., Ltd (agrochemical interests now Eiko Kasei Co., Ltd). Patents EP 48998; US 4377407; JP 6341393 Manufacturers Eiko Kasei
APPLICATIONS
Biochemistry Inhibits gibberellin biosynthesis. Mode of action Plant growth regulator which shortens lower internodes and upper leaf blades. Uses Used to increase resistance to lodging in rice, being applied to the soil surface under submerged conditions, at 1.5-2.4 kg/ha. Phytotoxicity Non-phytotoxic to rice. Formulation types GR; WP. Selected products: 'Seritard' (Eiko Kasei)
ANALYSIS
Product and residue analysis by hplc.
MAMMALIAN TOXICOLOGY
Reviews J. Pestic. Sci., 13, 391-394 (1988). Oral Acute oral LD50 for rats and mice >15 000 mg/kg. Skin and eye Acute percutaneous LD50 for rats and mice >5000 mg/kg. Not a skin or eye irritant to rabbits, nor skin sensitiser to guinea pigs. Inhalation LC50 (4 h) for rats >0.46 mg/l air. NOEL In 6 mo and 2 y toxicity studies on dogs and rats, no ill-effects were observed. In reproductive toxicity (3 generations) and teratogenicity studies on rats and rabbits, no abnormalities were observed. Other Not mutagenic in Ames assay. Toxicity class WHO (a.i.) U; EPA (formulation) IV
ECOTOXICOLOGY
Fish LC50 (48 h) for carp >30, grey mullet 11 mg/l. Daphnia LC50 (3 h) >30 mg/l.
ENVIRONMENTAL FATE
Animals The major urinary metabolite in the rat is 4-hydroxyinabenfide (H. Kinoshita et al.,Xenobiotica, 1987, 17, 925). Plants Metabolised to inabenfide ketone. Soil/Environment Half-life under Japanese paddy field conditions, c. 4 months.
|