|
imazalil
Fungicide
FRAC 3, G1; DMI: imidazole
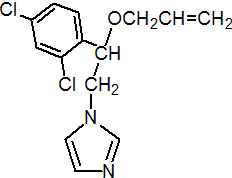
NOMENCLATURE
imazalil
Common name imazalil (BSI, E-ISO, (m) F-ISO, ANSI); chloramizol (Republic of South Africa); enilconazole (BAN, INN)
IUPAC name (?-1-(b-allyloxy-2,4-dichlorophenylethyl)imidazole; allyl (?-1-(2,4-dichlorophenyl)-2-imidazol-1-ylethyl ether
Chemical Abstracts name (?-1-[2-(2,4-dichlorophenyl)-2-(2-propenyloxy)ethyl]-1H-imidazole
CAS RN [35554-44-0] unstated stereochemistry; [73790-28-0] EEC no. 252-615-0 Development codes R023979 (for base); R018531 (for nitrate) (both Janssen)
imazalil sulfate
CAS RN [58594-72-2] EEC no. 261-351-5 (unspecified stereochemistry); 281-291-3 ((?- isomers) Development codes R027180 (Janssen)
PHYSICAL CHEMISTRY
imazalil
Mol. wt. 297.2 M.f. C14H14Cl2N2O Form Slightly yellow to brown, crystalline mass. M.p. 52.7 ºC B.p. >340 ºC V.p. 0.158 mPa (20 ºC) KOW logP = 3.82 (pH 9.2 buffer) Henry 2.61 ´ 10-4 Pa m3 mol-1 (calc.) S.g./density 1.348 g/ml (26 ºC) Solubility In water 0.18 g/l (pH 7.6, 20 ºC). In acetone, dichloromethane, ethanol, methanol, isopropanol, xylene, toluene, benzene >500, hexane 19 (all in g/l, 20 ºC). Also soluble in heptane and petroleum ether. Stability Very stable to hydrolysis in dilute acids and alkalis at room temperature, in the absence of light. Stable to temperatures up to 285 ºC. Stable to light under normal storage conditions. pKa 6.53, weak base F.p. 192 ºC
imazalil sulfate
Mol. wt. 395.2 M.f. C14H16Cl2N2O5S Form Almost white to beige-coloured powder. Solubility Freely soluble in water, alcohols, and slightly soluble in apolar organic solvents.
COMMERCIALISATION
History Fungicide reported by E. Laville (Fruits, 1973, 28, 545). Introduced by Janssen Pharmaceutica in 1977. Manufacturers Dow AgroSciences; Janssen
APPLICATIONS
Biochemistry Steroid demethylation (ergosterol biosynthesis) inhibitor. Mode of action Systemic fungicide, with protective and curative action. Uses Control of a wide range of fungal diseases on fruit, vegetables, and ornamentals, e.g. powdery mildews on cucurbits and ornamentals; powdery mildew on roses; storage diseases (particularly Penicillium, Gloeosporium, Phomopsis, Phoma spp., etc.) of citrus fruit, pome fruit, bananas, and seed potatoes. Also used as a seed dressing, for control of diseases (particularly Fusarium and Helminthosporium spp.) of cereals. It is particularly active against benzimidazole-resistant strains of plant-pathogenic fungi. Typical use rates are: for seed treatment 4-5 g/100 kg seed; for ornamentals and vegetables 5-30 g/hl; and for post-harvest treatment 2-4 g/t fruit. Formulation types EC; SP; Soluble liquid. Compatibility Incompatible with alkaline materials.
imazalil
Selected products: 'Deccozil' (Cerexagri); 'Flo-Pro' (Gustafson); 'Freshgard' (FMC); 'Magnate' (Makhteshim-Agan); 'Mazal' (Vapco); mixtures: 'Baytan Combi' (+ triadimenol+ fuberidazole) (fuberidazole not present in some formulations) (Bayer CropScience); 'Baytan Universal' (+ triadimenol+ fuberidazole) (seed treatment) (Bayer CropScience); 'Vincit' (+ thiabendazole+ flutriafol) (Cheminova)
imazalil sulfate
Selected products: 'Fungaflor' (Janssen); 'Fungazil' (Janssen); mixtures: 'Monceren IM' (+ pencycuron) (UK, Ireland) (Bayer CropScience)
OTHER PRODUCTS
imazalil
'Florasan' (Isagro); 'Nuzone' (Wilbur-Ellis); 'Sphinx' (Bayer CropScience); 'Stryper' (Crompton) mixtures: 'Baytan IM' (+ triadimenol+ fuberidazole) (Bayer CropScience); 'Citrocil' (+ 2-phenylphenol) (Citrosol); 'Extratect' (+ thiabendazole) (seed) (Syngenta); 'Manta Plus' (+ imidacloprid+ triadimenol+ fuberidazole) (Germany) (Bayer CropScience); 'Nectec' (+ azaconazole) (Hortichem, Janssen); 'Panoctine Plus' (+ guazatine) (Makhteshim-Agan); 'Raxil Complex Liquido' (+ tebuconazole) (seed treatment, Italy) (Bayer CropScience); 'Raxil IM' (+ tebuconazole) (seed treatment) (Bayer CropScience); 'Raxil MD Extra' (+ metalaxyl+ tebuconazole) (Gustafson); 'Raxil' (+ tebuconazole) (seed treatment, East Europe) (Bayer CropScience); 'Robust' (+ triticonazole) (Bayer CropScience, BASF); 'Vincit FS' (+ thiabendazole+ flutriafol) (Stähler, Cheminova); 'Vitaflo Extra' (+ thiabendazole+ carboxin) (Crompton); 'Vitavax Extra' (+ thiabendazole+ carboxin) (Crompton) Discontinued products mixtures: 'Larin' * (+ tebuconazole+ fenpiclonil) (seed treatment, Germany) (Syngenta); 'Raxil WS' * (+ tebuconazole) (seed treatment, E Europe) (Bayer)
imazalil sulfate
'Fecundal' (Janssen); 'Magnate Sulfate' (Makhteshim-Agan)
ANALYSIS
Product analysis by glc with FID (CIPAC Handbook, 1992, E, 95-100). Residues determined by glc (R. Woestenborghs et al., Med. Fac. Landbouwwet. Rijksuniv. Gent, 1988, 53, 1425) or by hplc (G. R. Cayley et al., Pestic. Sci., 1981, 12, 103).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 89, 91, 92, 94 (see part 2 of the Bibliography).
imazalil
Oral Acute oral LD50 for rats 227-343, dogs >640 mg/kg. Skin and eye Acute percutaneous LD50 for rats 4200-4880 mg/kg. Inhalation LC50 (4 h) for rats 16 mg/l air (with 200 g/l EC). NOEL for rats and dogs 2.5 mg/kg b.w. daily. ADI (JMPR) 0.03 mg/kg [2000, 2001]. Toxicity class WHO (a.i.) II; EPA (formulation) II EC classification Xn; R20/22| Xi; R41| N; R50, R53
imazalil sulfate
EC classification Xn; R20/22| Xi; R41| N; R50, R53
ECOTOXICOLOGY
imazalil
Birds LD50 for ring-necked pheasants 2000 mg/kg. LC50 (8 d) for mallard ducks >2510 mg/kg b.w. Fish LC50 (96 h) for rainbow trout 1.5, bluegill sunfish 4.04 mg/l. Daphnia LC50 (48 h) 3.5 mg/l. Bees Not hazardous to bees when used as directed. LD50 (oral) 40 mg/bee.
ENVIRONMENTAL FATE
Animals In rats, following oral administration, 90% is eliminated in the metabolised form within 4 days. Plants In plants, imazalil is transformed into a-(2,4-dichlorophenyl)-1H-imidazole-1-ethanol. Soil/Environment DT50 (field) 4-5 d; DT90 (field) 54-68 d. Soil adsorption K 182 (clay loam), 209 (sandy loam), 68 (sandy soil).
|