|
hexaconazole
Fungicide
FRAC 3, G1; DMI: triazole
NOMENCLATURE
Common name hexaconazole (BSI, ANSI, draft E-ISO, (m) draft F-ISO)
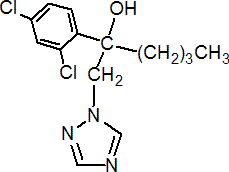
IUPAC name (RS)-2-(2,4-dichlorophenyl)-1-(1H-1,2,4-triazol-1-yl)hexan-2-ol
Chemical Abstracts name (?-a-butyl-a-(2,4-dichlorophenyl)-1H-1,2,4-triazole-1-ethanol
CAS RN [79983-71-4] Development codes PP523; ICIA0523 (both ICI)
PHYSICAL CHEMISTRY
Composition Tech. is >85% pure. Mol. wt. 314.2 M.f. C14H17Cl2N3O Form White, crystalline solid. M.p. 110-112 ºC V.p. 0.018 mPa (20 ºC) KOW logP = 3.9 (20 ºC) Henry 3.33 ´ 10-4 Pa m3 mol-1 (calc.) S.g./density 1.29 g/cm3 (25 ºC) Solubility In water 0.017 g/l (20 ºC). In dichloromethane 336, methanol 246, acetone 164, ethyl acetate 120, toluene 59, hexane 0.8 (all in g/l, 20 ºC). Stability Stable for at least 6 years at ambient temperatures. Stable to hydrolysis and photolysis in water. Formulations stable in sales containers for 6 months at 50 ºC, and 2 years at ambient temperature.
COMMERCIALISATION
History Fungicide introduced by ICI Agrochemicals (now Syngenta AG) and first marketed in 1986. Manufacturers Rallis; Sannong; Sharda; Syngenta
APPLICATIONS
Biochemistry Inhibits ergosterol biosynthesis (steroid demethylation inhibitor). Mode of action Systemic fungicide with protective and curative action. Uses Control of many fungi, particularly Ascomycetes and Basidiomycetes, e.g. Podosphaera leucotricha and Venturia inaequalis on apples, Guignardia bidwellii and Uncinula necator on vines, Hemileia vastatrix on coffee, and Cercospora spp. on peanuts, at 15-250 g/ha. Also used on bananas, cucurbits, peppers and other crops. Phytotoxicity Non-phytotoxic when used as directed. Some injury noted on McIntosh apples. Formulation types OL; SC; SG. Selected products: 'Anvil' (fruit) (Syngenta); 'Planete' (cereals) (Syngenta); 'Canvil' (Vapco); 'Contaf' (Rallis); 'Elexa' (Cequisa); 'Force' (Devidayal); mixtures: 'Columbia' (+ fenpropidin) (Syngenta)
OTHER PRODUCTS
'Proseed' (Syngenta); 'Blin' (IQV); 'Hexol' (Excel) mixtures: 'Acanto Dos' (+ picoxystrobin) (cereals) (Syngenta); 'Amistar Ter' (+ azoxystrobin) (cereals, peas) (Syngenta); 'Lynx' (+ chlorothalonil) (Syngenta); 'Sirius' (+ chlorothalonil) (Syngenta); 'Oscar' (+ bupirimate) (Makhteshim-Agan) Discontinued products: 'Bullet 5' * (Agro Chemicals) mixtures: 'Halley' * (+ ethirimol) (Zeneca)
ANALYSIS
Product by glc and FID. Residues by glc with either NPD, thermionic or ECD detection. See K. J. Harradine et al., in Comp. Anal. Profiles, Chapt. 2.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 59, 61 (see part 2 of the Bibliography). Oral Acute oral LD50 for male rats 2189, female rats 6071 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Mild irritant to eyes; non-irritating to skin (rabbits). Moderate skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >5.9 mg/l. NOEL NOAEL (2 y) for rats 10 mg/kg diet; NOAEL (2 y) for mice 40 mg/kg diet. ADI (JMPR) 0.005 mg/kg b.w. [1990]. Other Non-mutagenic. Toxicity class WHO (a.i.) U; EPA (formulation) IV EC classification Xn; R22| R43| N; R51, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >4000 mg/kg. Fish LC50 (96 h) for rainbow trout 3.4, mirror carp 5.94, sheepshead minnow 5.4 mg/l. Daphnia LC50 (48 h) 2.9 mg/l. Bees Acute oral and contact LD50 for honeybees >0.1 mg/bee. Worms LC50 (14 d) 414 mg/kg.
ENVIRONMENTAL FATE
Animals Readily excreted by mammals, with no significant retention in organs or tissues. Plants For details of the metabolism of hexaconazole in cereals, see M. W. Skidmore et al., Br. Crop Prot. Conf. - Pests Dis., 1990, 3, 1035-1040. Soil/Environment Rapidly degraded in soils in laboratory tests.
|