|
flutriafol
Fungicide
FRAC 3, G1; DMI: triazole
NOMENCLATURE
Common name flutriafol (BSI, ANSI, draft E-ISO, (m) draft F-ISO)
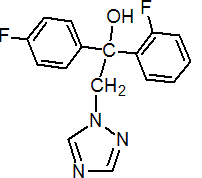
IUPAC name (RS)-2,4'-difluoro-a-(1H-1,2,4-triazol-1-ylmethyl)benzhydryl alcohol
Chemical Abstracts name (?-a-(2-fluorophenyl)-a-(4-fluorophenyl)-1H-1,2,4-triazole-1-ethanol
CAS RN [76674-21-0] Development codes PP450 (ICI)
PHYSICAL CHEMISTRY
Mol. wt. 301.3 M.f. C16H13F2N3O Form White, crystalline solid. M.p. 130 ºC V.p. 7.1 ´ 10-6 mPa (20 ºC) KOW logP = 2.3 (20 ºC) Henry 1.65 ´ 10-8 Pa m3 mol-1 (calc.) S.g./density 1.41 g/ml (25 ºC) Solubility In water 130 mg/l (pH 7, 20 ºC). In acetone 190, dichloromethane 150, methanol 69, xylene 12, hexane 0.3 (all in g/l, 20 ºC).
COMMERCIALISATION
History Fungicide reported by A. M. Skidmore et al. (Proc. 10th Int. Congr. Plant Prot., 1983, 1, 368) and P. J. Northwood et al. (ibid., 1983, 3, 930). Introduced by ICI Plant Protection Division (now Syngenta AG) and first marketed in 1983. Sold to Cheminova in 2001. Patents EP 15756
APPLICATIONS
Biochemistry Inhibits ergosterol biosynthesis (steroid demethylation inhibitor), causing fungal cell wall collapse and inhibition of hyphal growth. Mode of action Contact and systemic fungicide with eradicant and protective action. Absorbed by the foliage, with translocation acropetally in the xylem. Uses Control of a broad spectrum of leaf and ear diseases (including Erysiphe graminis, Rhynchosporium secalis, and Septoria, Puccinia, and Helminthosporium spp.) in cereals, at 125 g/ha. Also used in non-mercurial seed treatment formulations to control the major soil-borne and seed-borne diseases of cereals. Formulation types SC. Selected products: 'Impact' (Cheminova); mixtures: 'Vincit' (+ imazalil+ thiabendazole) (Cheminova)
OTHER PRODUCTS
'Atout' (Stähler, Cheminova); 'Cicero' (Cheminova); 'Pointer' (Cheminova) mixtures: 'Early Impact' (+ carbendazim) (Cheminova); 'Halo' (+ chlorothalonil) (Cheminova); 'Impact Excel' (+ chlorothalonil) (Cheminova); 'Pacer' (+ carbendazim) (Cheminova); 'Palette' (+ carbendazim) (Cheminova); 'Prospa' (+ chlorothalonil) (Cheminova); 'Vincit FS' (+ imazalil+ thiabendazole) (Stähler, Cheminova) Discontinued products mixtures: 'Ferrax' * (+ thiabendazole+ ethirimol) (seed) (Syngenta, Cheminova); 'Pacer' * (+ carbendazim) (Zeneca); 'Palette' * (+ carbendazim) (Zeneca); 'PP 375' * (+ chlorothalonil) (Zeneca)
ANALYSIS
Residues by glc; details from Syngenta.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 1140, female rats 1480 mg/kg. Skin and eye Acute percutaneous LD50 for rats >1000, rabbits >2000 mg/kg. Mild eye irritant; non-irritating to skin (rabbits). Not a skin sensitiser. Inhalation LC50 (4 h) for rats >3.5 mg/l. NOEL In 90 d feeding trials, rats receiving 2 mg/kg diet, and dogs receiving 5 mg/kg daily showed no ill-effects. Non-teratogenic in rats and rabbits. Other Non-cytogenic in in vivo studies. Non-mutagenic in the Ames assay. Toxicity class WHO (a.i.) III; EPA (formulation) III EC classification (Xn; R20/21/22| R52, R53)
ECOTOXICOLOGY
Birds Acute oral LD50 for female mallard ducks >5000 mg/kg. Dietary LC50 (5 d) for mallard ducks 3940, Japanese quail 6350 mg/kg. Fish LC50 (96 h) for rainbow trout 61, mirror carp 77 mg/l. NOEC 6.2 mg/l. Daphnia LC50 (48 h) 78 mg/l. Bees Low toxicity to honeybees. Acute oral LD50 >5 mg/bee. Worms LC50 (14 d) >1000 mg/kg.
ENVIRONMENTAL FATE
Soil/Environment Flutriafol has no effect on microbial populations, or on the carbon or nitrogen transformations occurring in the soil.
|