|
flurprimidol
Plant growth regulator
pyrimidinyl carbinol
NOMENCLATURE
Common name flurprimidol (BSI, draft E-ISO, (m) draft F-ISO, ANSI)
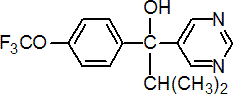
IUPAC name (RS)-2-methyl-1-pyrimidin-5-yl-1-(4-trifluoromethoxyphenyl)propan-1-ol
Chemical Abstracts name a-(1-methylethyl)-a-[4-(trifluoromethoxy)phenyl]-5-pyrimidinemethanol
CAS RN [56425-91-3] unstated stereochemistry Development codes EL-500 (Lilly)
PHYSICAL CHEMISTRY
Mol. wt. 312.3 M.f. C15H15F3N2O2 Form Colourless crystals. M.p. 93.5-97 ºC B.p. 264 ºC V.p. 4.85 ´ 10-2 mPa (25 °C) KOW logP = 3.34 (pH 7, 20 ºC) S.g./density 1.34 (24 ºC) Solubility In water 114 (distilled), 104 (pH 5), 114 (pH 7), 102 (pH 9) (all in mg/l, 20 ºC) (OECD 105). In hexane 1.26, toluene 144, dichloromethane 1810, methanol 1990, acetone 1530, ethyl acetate 1200 (all in g/l). Stability After 5 d at pH 4, 7 and 9 (50 ºC), <10% hydrolysis had occurred. Stable for at least 14 mo at room temperature. Photolytically decomposed in water, DT50 c. 3 h.
COMMERCIALISATION
History Plant growth regulator reported by G. E. Brown et al. (Abstr. Weed Sci. Soc. Am., No. 84, 1981). Introduced in USA (1989) by Eli Lilly & Co. (agrochemical interests now Dow AgroSciences). Rights acquired by SePRO Corp. in 2001. Patents US 4002628 Manufacturers SePRO
APPLICATIONS
Biochemistry Gibberellin synthesis inhibitor. Mode of action Plant growth regulator, absorbed by the leaves and roots, with translocation in the xylem and phloem. Reduces internode elongation. Uses Used to decrease the rate of growth in a wide range of mono- and dicotyledonous species, including perennial turf grasses, ornamental cover species, herbaceous and woody ornamentals, and deciduous and coniferous trees. Application rate for turf grasses is 0.125-1 lb/a; foliar rates for bedding plants are 2-80 ppm, for flowering and foliage plants 5-30 ppm, for woody ornamentals 100-200 ppm. Formulation types EC; SC; WP. Selected products: 'Cutless' (Sepro)
OTHER PRODUCTS
'Greenfield' (Sepro); 'Topflor' (Sepro)
ANALYSIS
Product analysis by glc with FID, or by hplc with u.v. detection. Residues in water or soils determined by glc with ECD, or by gc-ms (S. D. West in Comp. Anal. Profiles, Chapt. 9; S. D. West & B. S. Rutherford, J. Assoc. Off. Anal. Chem., 1986, 69, 572).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 914, female rats 709, male mice 602, female mice 702 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >5000 mg/kg. Slight to moderate irritant to skin and eyes (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >5 mg/l. NOEL (1 y) for dogs 7 mg/kg b.w. daily; (2 y) for rats 4, mice 1.4 mg/kg b.w. daily. ADI Not used on food crops. Other No teratogenic effects observed at doses of 200 mg/kg daily (rats) or 45 mg/kg daily (rabbits). Negative in Ames, DNA repair, primary rat hepatocyte and other in vitro assays. Toxicity class WHO (a.i.) III
ECOTOXICOLOGY
Birds Acute oral LD50 for quail and mallard ducks >2000 mg/kg. Dietary LC50 (5 d) for quail 560, mallard ducks 1800 mg/kg diet. Fish LC50 (96 h) for bluegill sunfish 17.2, rainbow trout 18.3 ppm. Daphnia LC50 (48 h) 11.8 ppm. Algae EC50 for Selenastrum capricornutum 0.84 mg/l. Bees LD50 (contact, 48 h) >100 mg/bee.
ENVIRONMENTAL FATE
Animals In mammals, the skin forms a significant barrier to absorption. Following oral administration, excretion follows in the urine and faeces within 48 hours, and more than 30 metabolites have been identified. No accumulation potential. Soil/Environment Degradation in soil under aerobic conditions leads to more than 30 metabolites. Soil Kd 1.7 on sandy loam.
|