|
flumioxazin
Herbicide
HRAC E WSSA 14; N-phenylphthalimide
NOMENCLATURE
Common name flumioxazin (BSI, draft ISO, ANSI)
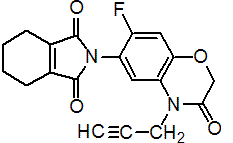
IUPAC name N-(7-fluoro-3,4-dihydro-3-oxo-4-prop-2-ynyl-2H-1,4-benzoxazin-6-yl)cyclohex-1-ene-1,2-dicarboxamide
Chemical Abstracts name 2-[7-fluoro-3,4-dihydro-3-oxo-4-(2-propynyl)-2H-1,4-benzoxazin-6-yl]-4,5,6,7-tetrahydro-1H-isoindole-1,3(2H)-dione
CAS RN [103361-09-7] Development codes S-53482 (Sumitomo); V-53482 (Valent)
PHYSICAL CHEMISTRY
Mol. wt. 354.3 M.f. C19H15FN2O4 Form Yellow-brown powder. M.p. 202-204 ºC V.p. 0.32 mPa (22 ºC) KOW logP = 2.55 (20 °C) S.g./density 1.5136 (20 ºC) Solubility In water 1.79 g/l (25 ºC). Soluble in common organic solvents. Stability Hydrolysis DT50 4.2 d (pH 5), 1 d (pH 7), 0.01 d (pH 9). Stable under normal storage conditions.
COMMERCIALISATION
History Reported by R. Yoshida et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1991, 1, 69). Introduced by Sumitomo. First registered in USA in 2001. Patents US 4640707; EP 170191 Manufacturers Sumitomo
APPLICATIONS
Biochemistry Protoporphyrinogen oxidase inhibitor. Acts, in the presence of light and oxygen, by inducing massive accumulation of porphyrins, and enhancing peroxidation of membrane lipids, which leads to irreversible damage of the membrane function and structure of susceptible plants. Mode of action Herbicide, absorbed by foliage and germinating seedlings. Uses Control of many annual broad-leaved weeds and some annual grasses pre- and post-emergence in soya beans, peanuts, orchards and other crops. Applied at 1-3 oz/a. Phytotoxicity Soya beans and peanuts are tolerant. Maize, wheat, barley and rice are moderately tolerant. Formulation types WG; WP. Selected products: 'Sumisoya' (Sumitomo)
OTHER PRODUCTS
'Pledge' (Sumitomo); 'Sumimax' (Sumitomo); 'Valor' (Valent) mixtures: 'Donjon' (+ diuron) (Sumitomo, Philagro); 'Groundboy' (+ glufosinate-ammonium) (Sumitomo)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin; mild eye irritant (rabbits). Non-sensitising to skin (guinea pigs). Inhalation LC50 (4 h) for rats >3930 mg/m3 air. NOEL Chronic dietary NOAEL 2 mg/kg daily. Other Non-mutagenic in the Ames test, in vivo chromosomal aberration, and in vivo/vitro UDS. EC classification R61| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2250 mg/kg. Dietary LC50 (5 d) for bobwhite quail and mallard ducks >5620 ppm. Fish LC50 (96 h) for rainbow trout 2.3, bluegill sunfish >21, sheepshead minnow >4.7 mg/l. Daphnia EC50 (48 h) for Daphnia pulex 5.5 ppm. Other aquatic spp. LC50/EC50 (96 h) for easter oyster 2.4, mysid shrimp 0.23 ppm. Bees LD50 for honey bees >105 mg/bee.
ENVIRONMENTAL FATE
Soil/Environment DT50 for photolysis on soil 5.8 d, for aerobic soil metabolism 14.7 d,
|