|
flumiclorac-pentyl
Herbicide
HRAC E WSSA 14; N-phenylphthalimide
NOMENCLATURE
flumiclorac-pentyl
Common name flumiclorac-pentyl (BSI, pa ISO, ANSI)
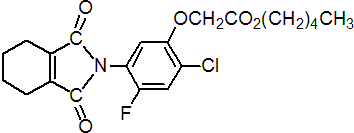
IUPAC name pentyl [2-chloro-5-(cyclohex-1-ene-1,2-dicarboximido)-4-fluorophenoxy]acetate
Chemical Abstracts name pentyl [2-chloro-4-fluoro-5-(1,3,4,5,6,7-hexahydro-1,3-dioxo-2H-isoindol-2-yl)phenoxy]acetate
CAS RN [87546-18-7] Development codes S-23031 (Sumitomo); V-23031 (Valent)
flumiclorac
Common name flumiclorac (BSI, pa E-ISO, ANSI)
IUPAC name [2-chloro-5-(cyclohex-1-ene-1,2-dicarboximido)-4-fluorophenoxy]acetic acid
Chemical Abstracts name [2-chloro-4-fluoro-5-(1,3,4,5,6,7-hexahydro-1,3-dioxo-2H-isoindol-2-yl)phenoxy]acetic acid
CAS RN [87547-04-4]
PHYSICAL CHEMISTRY
flumiclorac-pentyl
Mol. wt. 423.9 M.f. C21H23ClFNO5 Form Beige solid, with a halide odour. M.p. 88.9-90.1 ºC V.p. <0.01 mPa (22.4 ºC) KOW logP = 4.99 (20 ºC) S.g./density 1.33 g/ml (20 ºC) Solubility In water 0.189 mg/l (25 ºC). In methanol 47.8, hexane 3.28, n-octanol 16.0, acetone 590 (all in g/l). Stability Hydrolytic DT50 4.2 d (pH 5), 19 h (pH 7), 6 min (pH 9). Aqueous photolysis DT50 are similar. F.p. 68 ºC
flumiclorac
Mol. wt. 353.7 M.f. C16H13ClFNO5
COMMERCIALISATION
Patents US 4770695; EP 83055 Manufacturers Sumitomo
APPLICATIONS
flumiclorac-pentyl
Biochemistry Protoporphyrinogen oxidase inhibitor. It is suggested that flumiclorac-pentyl induces accumulation of porphyrins in susceptible plants. The photosensitising action of accumulated porphyrins is likely to be one of the causes of membrane lipid peroxidation, which in turn leads to irreversible damage of membrane function and structure in susceptible plants, and results in symptoms outlined below. Differences in metabolism of flumiclorac-pentyl seem to be the basis of crop selectivity. Experiments with 14C-flumiclorac-pentyl suggest more rapid degradation in soya beans than in Abutilon. Mode of action Fast-acting, contact post-emergence herbicide. When applied to foliage of susceptible plants, it is readily absorbed into plant tissue and causes characteristic herbicidal symptoms such as desiccation, wilting, bleaching, browning and necrosis. Uses Pre- and post-emergence herbicide for control of problem broad-leaved weeds, including Xanthium strumarium, Chenopodium album, Ambrosia artemisiifolia, Datura stramonium, Amaranthus spp., Sida spinosa, Euphorbia maculata, and Abutilon theophrasti, in soya beans and maize, at 0.027-0.054 lb/a. Formulation types EC. Selected products: 'Resource' (Sumitomo, Valent); 'Sumiverde' (Sumitomo)
OTHER PRODUCTS
flumiclorac-pentyl
Mixtures: 'Stellar' (+ lactofen) (Sumitomo, Valent)
MAMMALIAN TOXICOLOGY
flumiclorac-pentyl
Oral Acute oral LD50 for rats 3.69 kg 0.86EC/kg. Skin and eye Acute percutaneous LD50 for rabbits >2.0 g 0.86EC/kg. Moderately irritating to skin and eyes; not a skin sensitiser. Inhalation LC50 for rats 5.51 mg 0.86EC/l.
ECOTOXICOLOGY
flumiclorac-pentyl
Birds Acute oral LD50 for bobwhite quail >2250 mg/kg. Dietary LC50 for bobwhite quail and mallard ducks >5620 ppm. Fish LC50 for rainbow trout 1.1, bluegill sunfish 13-21 mg/l. Daphnia LC50 (48 h) >38.0 mg/l. Bees LD50 (contact) >196 mg/bee.
ENVIRONMENTAL FATE
Plants In soya beans and maize, the major metabolite is 2-chloro-4-fluoro-5-(4-hydroxy-1,2-cyclohexanedicarboximido)phenoxyacetic acid formed by reduction of the tetrahydrophthaloyl double bond and hydroxylation; other metabolic pathways include cleavage of the ester, and cleavage of the imide linkage. Soil/Environment Rapidly degraded in soil: DT50 0.48-4.4 d in loamy-sand soil (pH 7); degradates have DT50 c. 2-30 d. The a.i. is immobile in soil; degradates have low to medium mobility. No residues of parent or degradates were observed below the upper 3 inches of soil.
|